CT/MRI Liver Imaging Reporting and Data System (LI-RADS) is an algorithm for diagnosing and staging hepatocellular carcinoma (HCC) (CT/MRI Diagnostic LI-RADS) or assessing the response of HCC to locoregional treatment (CT/MRI Treatment Response LI-RADS) using CT or MRI with extracellular contrast agents or MRI with hepatobiliary contrast agents3.
The current version is CT/MRI LI-RADS v2018 with a separate CT/MRI Treatment Response LI-RADS v2024 expected in late 2024 3. The article below relates specifically to CT/MRI Diagnostic LI-RADS v2018. For other LI-RADS algorithms see: LI-RADS (overview).
On this page:
Terminology
Focal abnormalities are referred to as "observations" in LI-RADS rather than lesions or nodules, as each observation may not be a true lesion or nodule but could reflect perfusion abnormalities or distortions of the background liver.
If an observation has been biopsied and the diagnosis is certain, the pathological diagnosis should be reported rather than the LI-RADS category. If the observation has been biopsied but the diagnosis is uncertain or it is an HCC precursor (regenerative or dysplastic nodule), the LI-RADS and the pathology diagnosis should be reported together.
Major criteria
-
non-rim arterial phase hyperenhancement (APHE)
hyperenhancement: enhancement in the arterial phase is definitely greater than that of background liver
if unsure, classify as iso-enhancing
-
nonperipheral "washout"
a visual assessment of relative hypointensity of the lesion compared with background liver on the portal venous and delayed phases
with gadoxetate there must be hypointensity in the portal venous phase
-
enhancing "capsule"
peripheral rim of smooth hyperenhancement seen in the portal venous phase, transitional phase, or delayed phase
-
size
largest outer edge to edge dimension
should include "capsule" in measurement
measured on the phase or sequence in which margins are best seen
measurement on arterial phase or DWI is discouraged if margins better seen on other phases, as size on these may be over estimated
-
threshold growth
-
diameter increase ≥50% increase in ≤6 months
-
other prior criteria are now considered subthreshold growth, an ancillary feature
if prior exam >6 months, diameter ≥100% increase
a new lesion ≥10 mm in <24 months
-
threshold growth should be compared on similar sequences between studies
only apply this criterion if the lesion is definitely a mass (e.g. not perfusion alteration)
-
Ancillary features
-
favouring malignancy, not HCC in particular
ultrasound visibility as a discrete nodule
subthreshold growth (see "threshold growth" in the major criteria section)
corona enhancement
fat sparing in a solid mass
restricted diffusion
mild-to-moderate T2 hyperintensity
iron sparing in a solid mass
transitional phase hypointensity
hepatobiliary phase hypointensity
-
favouring HCC in particular
nonenhancing "capsule"
nodule-in-nodule architecture
mosaic architecture
fat in mass, more than adjacent liver
blood products in mass
-
favouring benignity
size stability ≥2 years
size reduction
homogeneous marked T2 hyperintensity
homogeneous marked T2 or T2* hypointensity
undistorted vessels
parallels blood pool enhancement
hepatobiliary phase isointensity
Classification
Major criteria imaging findings often lead directly to the assignment of the LI-RADS score. If the assignment is unclear, ancillary findings may be useful as a "tie-breaker".
The LI-RADS score ranges from LR-1 (favour benignity) to LR-5 (favour malignancy).
LR-1 (100% benign)
-
imaging features diagnostic of a benign entity:
vascular anomaly
perfusion alteration
hypertrophic pseudomass
focal scar
definite disappearance at follow-up in the absence of treatment is also indicative of LR-1
LR-2 (probably benign)
-
entities are similar to LR1, but the appearance is highly suggestive of the entity instead of 100% diagnostically certain
atypical appearance of benign entities may be categorised as LR2
LR2 cirrhosis-associated nodule is also included
LR-3 (intermediate probability for HCC)
not a definitely benign entity, but not definitely HCC
-
includes entities with the following features:
not a definite mass
-
mass with hepatic arterial phase iso- or hypoenhancement
-
<20 mm with no more than one of the following:
nonperipheral "washout"
capsule
threshold growth
-
-
mass with hepatic arterial phase hyperenhancement
<20 mm with no "washout," capsule, or threshold growth
LR-4 (probably HCC)
-
no arterial phase hyperenhancement
-
<20 mm
-
two or more of the following
non-peripheral "washout"
enhancing capsule
threshold growth
-
-
≥20 mm
-
one or more of the following
non-peripheral "washout"
enhancing capsule
threshold growth
-
-
-
non-rim arterial phase hyperenhancement
-
<10 mm
-
one or more of the following
non-peripheral "washout"
enhancing capsule
threshold growth
-
-
10-19 mm
enhancing "capsule", but does not meet threshold growth or washout criteria
-
≥20 mm
-
no major suspicious features:
non-peripheral "washout"
enhancing capsule
threshold growth
-
-
LR-5 (100% definite HCC)
-
non-rim arterial phase hyperenhancement
-
10-19 mm
single major suspicious feature (washout or threshold growth), excluding enhancing "capsule" (LR-4)
-
two or more of the following
threshold growth
enhancing capsule
nonperipheral "washout"
-
≥20 mm
-
one or more of the following
threshold growth
enhancing capsule
nonperipheral "washout"
-
-
Special categories
LR-M for liver lesions that are probably or definitely malignant, but not an appearance compatible with HCC. Examples of this include:
targetoid mass
-
non-targetoid mass with
infiltrative appearance
marked diffusion restriction
necrosis or severe ischaemia
other appearance that in the radiologists' judgement suggests a non-HCC malignancy
LR-NC (LR-non-categorisable) for lesions in which the technical quality of the MRI does not allow evaluation of the major features.
LR-TIV for unequivocal enhancing soft tissue invading the portal vein, regardless of whether an underlying parenchymal mass is visible. This is important to report since it is a contraindication to liver transplantation. Malignancies other than HCC can invade the portal venous system.
Treatment and prognosis
Suggested management:
LR-1: continued routine surveillance
LR-2: continued routine surveillance, consider repeat diagnostic imaging in six months or less
LR-3: repeat or alternative diagnostic imaging in 3-6 months
LR-4: multidisciplinary team discussion for tailored workup, may include biopsy
LR-5: diagnosis confirmed - plan treatment
LR-NC: repeat or alternative diagnostic imaging in three months or less
Practical points
ancillary features can upgrade or downgrade a lesion one category but cannot upgrade a lesion to LR-5
if there are no LR-4 or LR-5 lesions, then LR-3 lesions should be reported, otherwise reporting of LR-3 lesions is at the radiologist's discretion (they should be reported if previously LR-4 or LR-5)
late hepatic arterial phase is preferred for evaluation of arterial hyperenhancement
for masses with nodule-in-nodule appearance, measure the entire mass


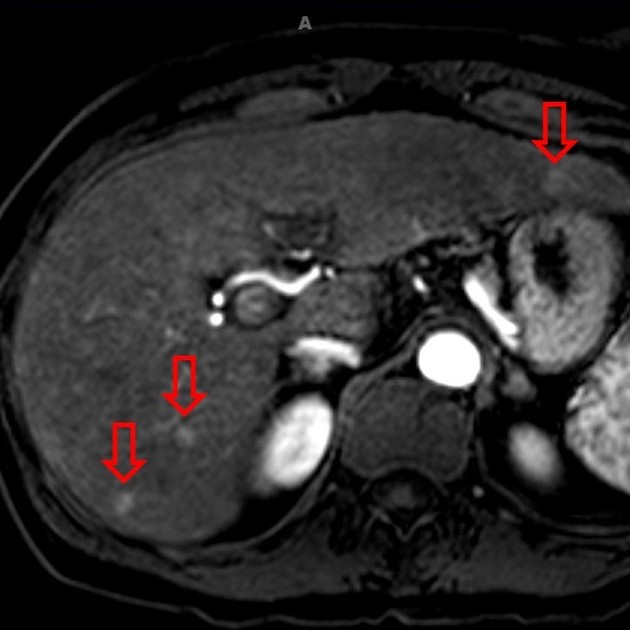
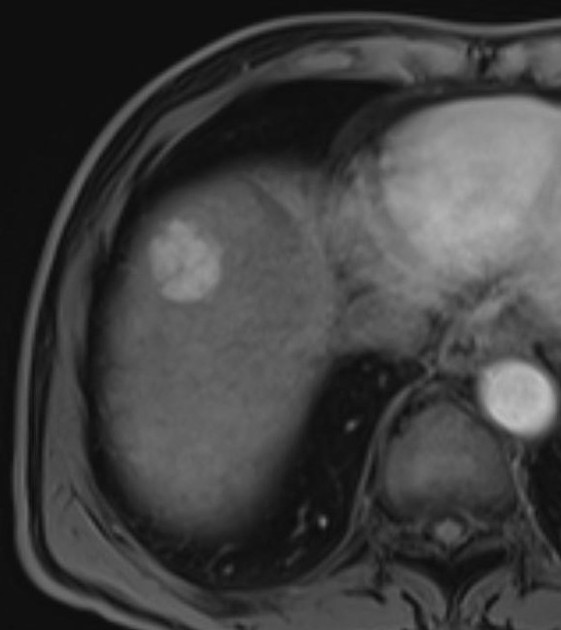
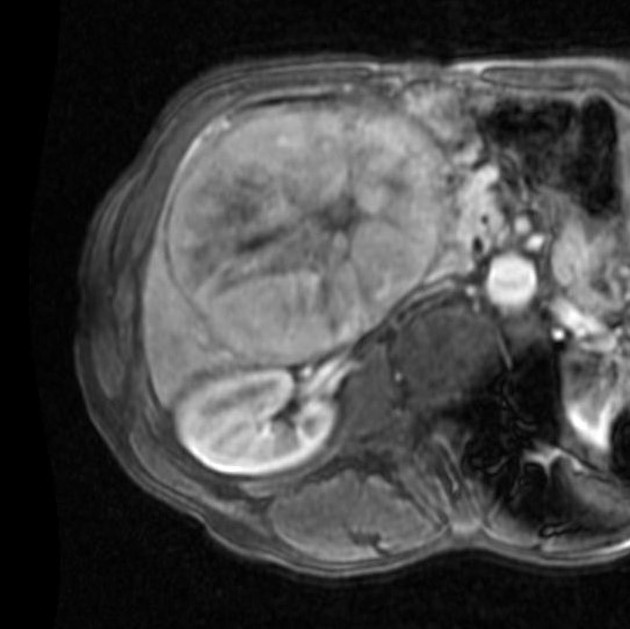
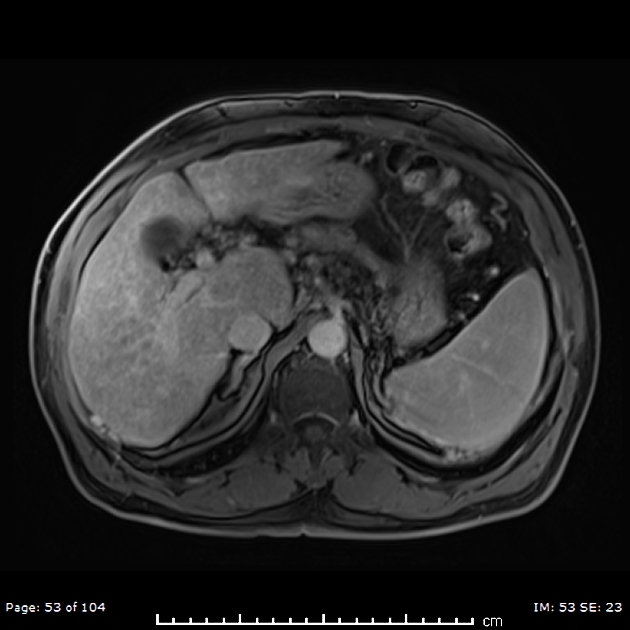


 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.