Polycystic ovarian syndrome (PCOS), also known as hyperandrogenic anovulation, is a chronic anovulation syndrome associated with androgen excess in women of reproductive age.
On this page:
Terminology
"Hyperandrogenic anovulation" has been proposed as a more accurate and potentially less confusing term, as the ovarian feature is of multiple follicles and not cysts 13. At this stage, however, "polycystic ovarian syndrome" remains the term that is widely known and used.
Diagnosis
The 2018 International Evidence-based Guideline criteria (based on the 2003 Rotterdam criteria, updated in 2023) 23 are used to make the diagnosis of PCOS after exclusion of other aetiologies (e.g. congenital adrenal hyperplasia, Cushing syndrome, and/or an androgen-secreting tumour) and require at least two of the following 4,18,23:
ovulatory dysfunction (oligo- and/or anovulation)
clinical and/or biochemical signs of hyperandrogenism
polycystic ovarian morphology (PCOM) on ultrasound (see Radiographic features) or elevated serum AMH
Hence, ultrasound is not necessary for the diagnosis if features of both ovulatory dysfunction and hyperandrogenism are present, or if one of these features is found in conjunction with an elevated serum AMH.
Epidemiology
The estimated prevalence is ~10% (range 8-13%) of women of reproductive age but this varies (up to 20%) depending on the diagnostic criteria used 11,23.
Clinical presentation
The classic triad of PCOS is:
oligomenorrhoea and/or anovulation
hirsutism
In addition to this, patients commonly have infertility, acne, alopecia or biochemically show increased androgen levels as well as pelvic pain being common 25. Hormone-related skin changes as patches of darkened skin can also occur 25.
Pathology
Markers
Biochemical hyperandrogenism is based on the measurement of free testosterone, free androgen index, or calculated bioavailable testosterone, androstenedione and dehydroepiandrosterone sulphate 20.
Anti-Müllerian hormone (AMH) levels are generally increased, and there is emerging evidence for the utility of AMH in the diagnosis of PCOS 23.
Associations
-
obesity and family history of obesity 25
obesity is present in up to 80% of women from the United States of America with polycystic ovarian syndrome, however, this association is not as strong outside of the United States of America 24
subfertility and recurrent pregnancy loss
-
long-term increased risk of
type 2 diabetes mellitus 25
hyperlipidaemia 25
cardiovascular disease 25
hypertension 25
endometrial cancer (two to six-fold increased risk) 6,18
high prevalence of anxiety and depression
women with polycystic ovarian morphology are at increased risk of ovarian hyperstimulation syndrome (OHSS) when undergoing IVF, regardless of whether they have PCOS 15
Radiographic features
Ultrasound
The ovaries may appear normal in PCOS, and conversely, polycystic ovarian morphology (PCOM) may be seen in women without the syndrome. However, it is well accepted that women with PCOS tend to have larger ovaries with an increased number of follicles ref.
The specific diagnostic cut-offs, however, have been the subject of debate and revision. The updated diagnostic criteria (c. 2024) are based on a 2023 international consensus guideline and supersede the 2003 Rotterdam criteria of ≥12 follicles and interim recommendations of ≥25 follicles 23.
In patients >8 years post menarche, the threshold for polycystic ovarian morphology (PCOM) is 23:
follicle number per ovary (FNPO) ≥20 in at least one ovary (considered the most accurate ultrasound finding)
or if image quality is insufficient for a complete follicle count then the threshold for polycystic ovarian morphology (PCOM) is at least one of the following 23:
follicle number per section (FNPS) ≥10 in at least one ovary, and/or
ovarian volume ≥10 mL, ensuring no corpora lutea, cysts or dominant follicles are present
FNPO should include any follicles measuring 2-9 mm. Other ovarian features and/or pathology including ovarian cysts, corpus lutea and dominant follicles ≥10 mm should not be included in ovarian follicle counts or volume calculations.
The diagnostic criteria are adjusted in adolescent females (defined as within 8 years of menarche, or age <20 years) 18,23, in whom ultrasound should not be used for the diagnosis of PCOS due to the high incidence of multi-follicular ovaries in this life stage 22.
Other morphological features have been described, but do not contribute to formal diagnostic criteria:
hyperechoic central stroma
peripheral location of follicles (string of pearls sign)
follicles of similar size measuring 2-9 mm
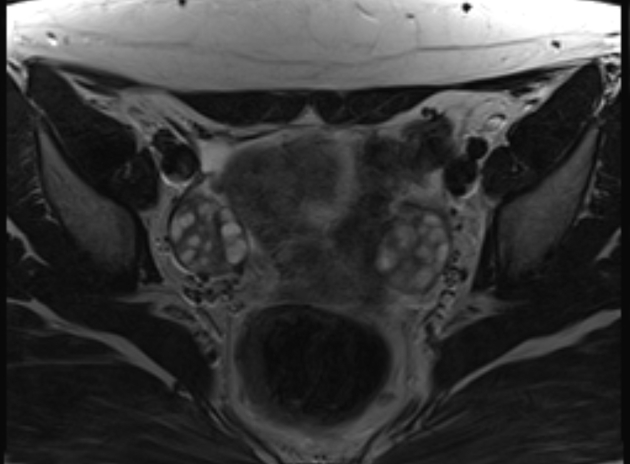
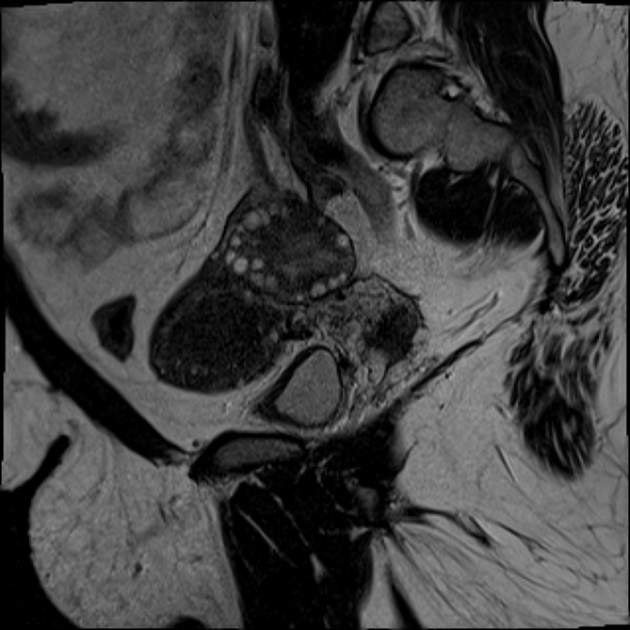
MRI
MRI is not warranted routinely in the investigation of PCOS, nonetheless, pelvic MRI may show most or all of the above sonographic features. Signal characteristics include:
T1: small uniform follicles are low in signal while the central stroma is of intermediate signal (vs normal myometrium)
T2: follicles have high T2 signal while the central stroma is of comparatively low T2 signal 8
History and etymology
The syndrome was first described by I F Stein and M L Leventhal in 1935 7.
Practical points
with a lack of consensus sometimes it is easier to report the number of follicles in each ovary rather than attempt to label the ovaries as "polycystic" or "multifollicular"
ultrasound should not be used for the diagnosis of PCOS in patients <8 years after menarche due to the high incidence of multi-follicular ovaries in this life stage
as the individual age of menarche may not be known, an age cut-off of 20 years is suggested for the utility of ultrasound in this diagnosis (based on the median age at menarche being approximately 12 years)
post menopause, a new or continuing diagnosis of PCOS could be considered based on past history and clinical evidence of persistent hyperandrogenism
postmenopausal women presenting with new-onset, severe or worsening hyperandrogenism including hirsutism, require further investigation to rule out androgen-secreting tumours and ovarian hyperthecosis






 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.