Uraemic encephalopathy is an acquired toxic syndrome characterised by delirium in patients with untreated or inadequately treated acute or chronic kidney disease 13. Uraemic encephalopathy is often associated with lethargy and confusion in the acute phase, which can progress to seizures, coma, or both in the chronic phase. Several neurochemical alterations have been reported in the acute and chronic phases of uraemic encephalopathy, including alterations in water transport and cerebral oedema, alterations in the blood-brain barrier, and changes in cerebral metabolism.
On this page:
Epidemiology
There are very few studies dealing with the epidemiology of the encephalopathic aspect of renal dysfunction. One study investigated cognitive impairment in patients on haemodialysis and found that among 374 patients on haemodialysis, 55 years or older, only 12.7% were completely cognitively intact. Almost 14% demonstrated mild cognitive impairment, 36.1% demonstrated moderate cognitive impairment, and 37.3% demonstrated severe cognitive impairment 1. Uraemic retention solutes, anaemia and hyperparathyroidism may play distinct roles in the pathogenesis of uraemic encephalopathy 2.
Clinical presentation
Uraemic encephalopathy presents as a complex syndrome which varies in its presentation from mild sensory clouding to delirium and coma, occasionally associated with myoclonus, asterixis, and seizures 3.
Pathology
Aetiology
Animal studies have demonstrated impressive activations of biogenic amine expressing cell groups, stress-sensitive areas, and cell groups involved in the regulation of water and electrolyte homoeostasis, as well as central autonomic cell groups, in surgically and medically induced renal failure in rats 3. Data reported indicated that the acute uraemic state is an important factor that influences a variety of neurochemicals such as the biogenic amines (noradrenaline, adrenaline, histamine, and 5-hydroxytryptamine (5-HT)), as well as various brain areas such as stress-sensitive forebrain areas, neuronal cell groups involved in the regulation of water and electrolyte homoeostasis, and central autonomic cell groups 3. Animal studies comparing acute uraemic encephalopathy with hepatic encephalopathy have demonstrated an increase in brain inflammation in conjunction with an increase in vascular permeability in uraemic encephalopathy 4. Local kidney injury may activate cytokines that cross the blood-brain barrier or activate other messengers that contribute to neuronal dysfunction. Alternatively, the retention of uraemic solutes may trigger both the inflammatory reaction and neuronal dysfunction 2.
Case reports and studies in humans have reported a number of biochemical changes in acute and chronic uraemic encephalopathy, including alterations in water transport and brain oedema, disturbances of the blood-brain barrier, and changes in cerebral metabolism 5,6. Anaemia, hyperparathyroidism and brain calcium concentrations have also been implicated in uraemic encephalopathy 7,8.
Macroscopic and microscopic features
Non-specific neuropathologic abnormalities have been described, including cerebral atrophy, gliosis, and foci of perivascular necrosis with accumulation of macrophages. Alzheimer type II astrocytes are often prominent. Patients may also develop changes of hypertensive encephalopathy 9.
Radiographic features
Uraemic encephalopathy is a syndrome in which the subcortical grey and white matter, midbrain and mesial temporal lobes become oedematous due to their exquisite sensitivity to metabolic alterations which is an inherent vulnerability related to the arterial perforators which supply these areas. The imaging features are thus consistent with cytotoxic oedema, localised in those areas.
CT
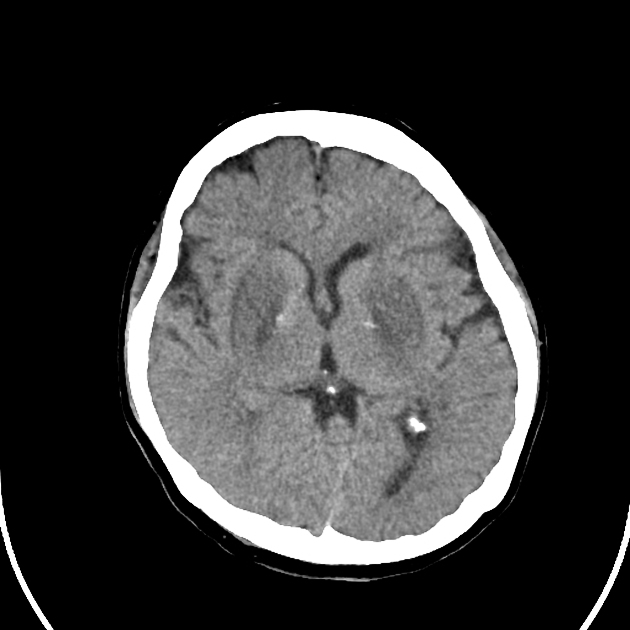
Uraemic encephalopathy on CT typically presents as confluent bilateral hypodensity involving the basal ganglia, thalamus and midbrain. The anatomical boundaries between the deep subcortical grey matter typically appear obliterated 10.
MRI
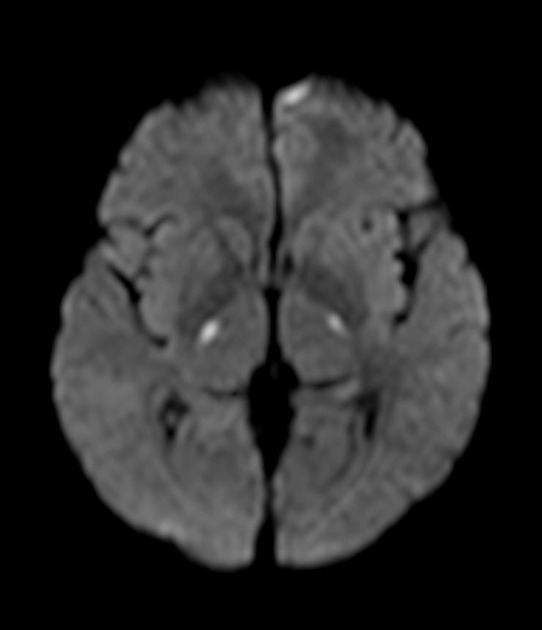
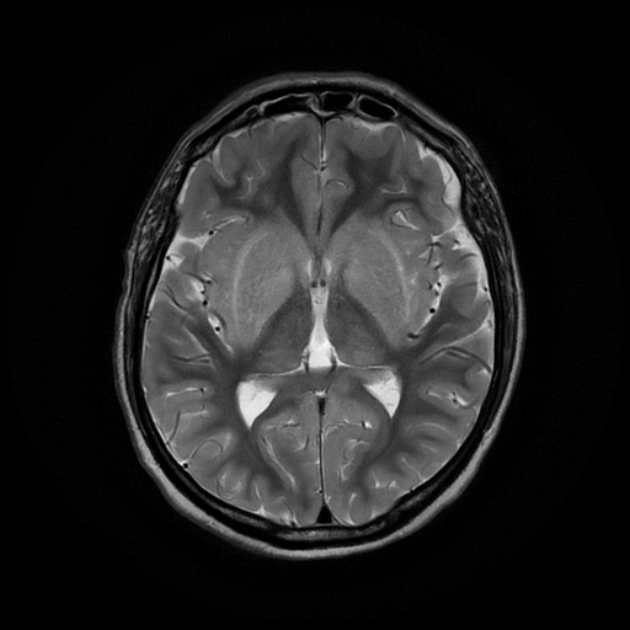
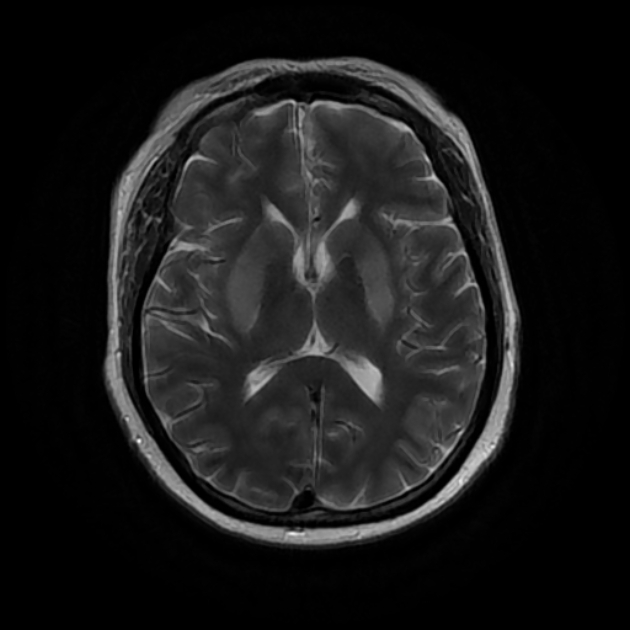
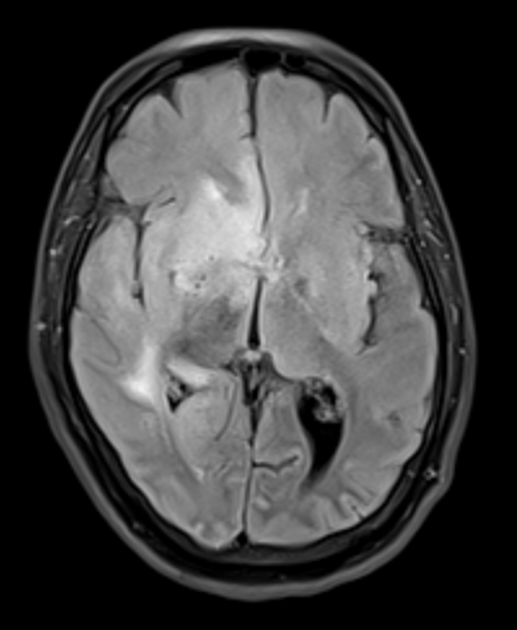
Uraemic encephalopathy typically presents as bilateral T2/FLAIR hyperintensities involving the basal ganglia, thalamus, midbrain and mesial temporal lobes. Restricted diffusion may or may not be present to a varying degree, but is not characteristic of uraemic encephalopathy. Enhancement is not a typical feature.
lentiform fork sign: is a characteristic feature of uraemic encephalopathy, in which the white matter surrounding the basal ganglia (the internal and external capsules and the medullary laminae) becomes hyperintense on T2/FLAIR
Treatment and prognosis
Dialysis is the primary treatment for uraemic encephalopathy. This may be preceded by a period of peritoneal dialysis, which can be administered to ambulatory patients. Many patients ultimately require renal transplantation. Epileptic seizures, including non-convulsive seizures, occur in up to one-third of all uraemic patients. In evaluating patients with seizures, it is essential to determine whether the seizure is the result of uraemia or the consequence of some other coexisting or causative illness such as malignant hypertension with encephalopathy, intercurrent infection, dialysis disequilibrium syndrome, or cerebral infarction. Usually, the seizures caused by uncomplicated uraemia are generalised, but focal motor seizures and epilepsia partialis continua occur 11.
Differential diagnosis
Other toxic encephalopathies such as acute liver failure and hepatic encephalopathy, metabolic disturbances such as disorders of glucose metabolism and disorders of water and electrolyte metabolism, and finally drug overdose and toxic exposures should also be considered.
The vast majority of toxic and metabolic disorders of the brain involve the deep grey nuclei (basal ganglia and thalamus) or the cerebral white matter. Typically, there is a symmetric abnormality of the involved structures, which can provide a clue to the correct diagnosis 12.






 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.