Presentation
A young male presented with cognitive decline, with mild fever for a week. No previous history of malignancy.
Patient Data
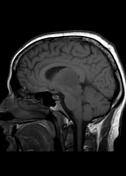
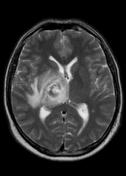
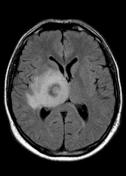
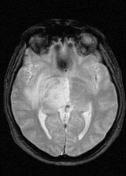
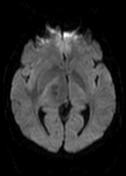
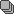
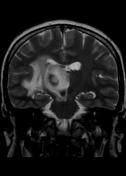
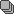
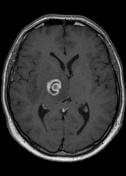
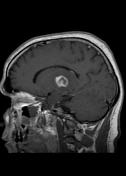
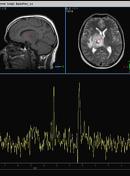
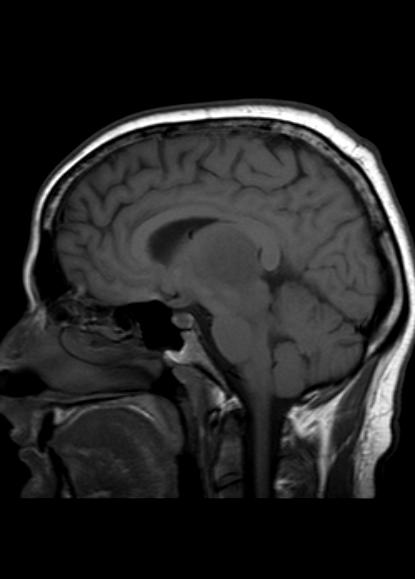
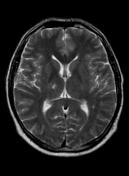
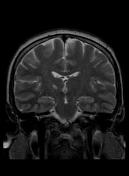
MR demonstrates right thalamic crescent-shaped, well-defined lesion with perilesional vasogenic oedema. The lesion is hypointense on T1WI, mildly hyperintense on T2WI, with dual rim best seen on T2WI. No calcifications or blood products within the lesion on T2GRE images. Diffusion-weighted images (DWI) shows hypointensity and no restriction. The lesion shows ring enhancement after IV contrast administration.
MR-spectroscopy shows a mild amino-acid peak (0.9 ppm) and severe elevation of the lipid/lactate peak (1.33 ppm). There is also marked depression of the NAA (2.03 ppm) and Creatine (3.02 ppm), no depression of the choline.
Brain MR and MRS findings are most keeping with cerebral toxoplasmosis and less likely brain abscess.
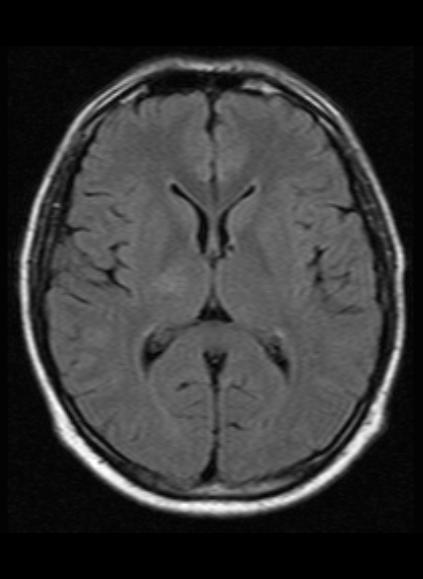
Laboratory and clinical tests reveal a positive HIV test. Tuberculosis markers were negative. The patient treated with broad-spectrum antibiotics.
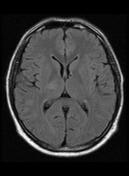
Follow up MRI after 5 months shows complete resolution of the right thalamic lesion with minimal T2 and FLAIR hyperintense gliotic changes.
Case Discussion
The case illustrates typical findings for cerebral toxoplasmosis.




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.