Alexander disease, also known as fibrinoid leukodystrophy, is a rare fatal leukodystrophy. Usually clinically evident in the infantile period, neonatal, juvenile, and adult variants are recognised. As with many diseases with a variable age of presentation, the earlier it manifests the more fulminant the clinical course.
On this page:
Classification
Traditionally, Alexander disease was divided into three forms based on age of onset, with neonatal being added later as a fourth distinct form 6:
neonatal onset: <30 days
infantile/childhood-onset (most common): 0-2 years
juvenile onset: 2-12 years
adult-onset (AOAD): >12 years
Alternatively, it can be divided according into two types 7:
-
type I: typical MRI features
early onset (usually <4 years of age)
encephalopathy, failure to thrive, macrocephaly, motor delay, seizures
-
type II: atypical MRI features
any age, but typically later-onset
autonomic dysfunction, bulbar symptoms, ocular movement abnormalities
Childhood-onset Alexander disease
Childhood-onset Alexander disease (usually type 1) 7 typically manifests in infants and adolescents. It is typically sporadic, although in some cases the gene for glial fibrillary acidic protein (GFAP) has been implicated.
Clinical presentation
Macrocephaly is usually present, with other clinical features include progressive quadriparesis and spasticity, seizures, and intellectual disability.
Pathology
Most cases are sporadic. Familial disease has also been reported, however. A heterozygous mutation in the coding region, mapped to chromosome 17q21, of GFAP, an astrocyte-specific intermediate filament protein, is associated with most cases of infantile sporadic onset.
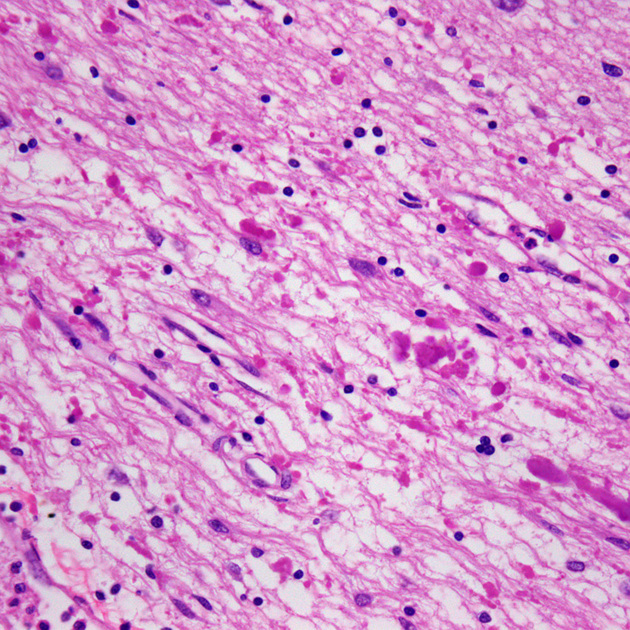
Histologically the disease is characterised by the accumulation of large numbers of Rosenthal fibres and eosinophilic granular bodies (large accumulations agglomerations of astrocytic processes) in the degenerated (demyelinated) white matter which is a product of GFAP. This is on its own a non-specific finding, as they are also seen in slow-growing or benign astrocytomas (e.g. pilocytic astrocytomas). Intracellular deposition of Rosenthal fibres may cause abnormal functioning of the oligodendrocytes.
Radiographic features
The disease begins in the frontal region and extends posteriorly. Subcortical U-fibres are somewhat initially spared but affected relatively early in the course of the disease. End-stage disease is characterised by contrast-enhancing cystic leukomalacia.
MRI
-
T2: increased signal in
bifrontal white matter which tends to be symmetrical
periventricular rim
T1 C+ (Gd): enhancement may be seen in the same areas
Obstructive hydrocephalus secondary to periaqueductal involvement and swelling of basal ganglia may be seen.
Juvenile/adult-onset Alexander disease
Adult-onset Alexander disease (AOAD), which was only recognised with any frequency after GFAP was recognised as the mutation, has markedly different imaging findings and presentation.
Clinical presentation
Adult-onset Alexander disease presents with prominent bulbar symptoms (dysphagia, dysphonia or dysarthria), pyramidal pattern of weakness and spasticity, cerebellar ataxia, symptomatic palatal tremor (palatal myoclonus), sleep disturbance and autonomic dysfunction 9,10.
Pathology
Adult-onset Alexander disease may have an autosomal pattern of inheritance in some pedigrees 2,14. Usually, this is an autosomal dominant pattern, but autosomal recessive forms have rarely been described 2,14.
Radiographic features
MRI
-
T2: increased T2 signal (representing demyelination) and atrophy affecting multiple regions
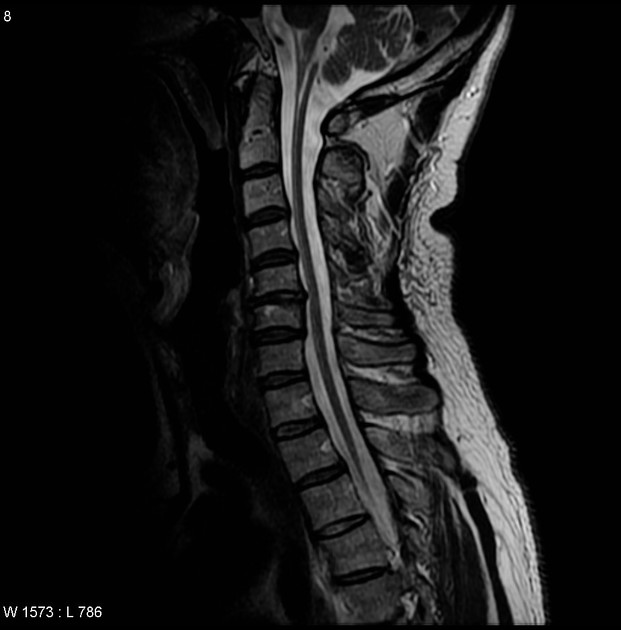
medulla and upper cervical cord: chipmunk sign of signal abnormalities in the medulla (axial), hot cross bun-like sign of signal abnormalities in the medulla (axial), and tadpole sign of atrophy (sagittal) 5,10-12
occasional involvement of inferior cerebellar peduncles
pons, middle and superior cerebellar peduncles (rare)
supratentorial (typically periventricular) may be seen in patients <40 years of age 10
T1 C+ (Gd): patchy enhancement uncommonly seen in adult patients
In addition to signal abnormalities and atrophy, presence of 'ventricular garlands' has also been described as a characteristic feature of adult-onset Alexander disease, which consist of a garland-like pattern of blood vessels (likely veins) and perivascular Rosenthal fibres, best seen along the inner surface of the lateral ventricles on T1 and/or T2 weighted sequences 12,13.
History and etymology
The condition is named after New Zealand pathologist William Stewart Alexander (1919-2013) who first described the condition in a 1949 case report 8.





 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.