The four-phase liver CT protocol is a useful examination in the assessment of focal liver lesions, hypervascular liver metastasis and endocrine tumours.
It is a triple-phase liver with an initial non-contrast component included before the intravenous contrast medium is given, often requested if patients have received an injection of drug-eluting beads such as Lipiodol.
It involves a non-contrast liver followed by a dedicated late arterial phase, portal venous phase and delayed phase acquisition.
NB: This article is intended to outline some general principles of protocol design. The specifics will vary depending on CT hardware and software, radiologists' and referrers' preference, institutional protocols, patient factors (e.g. allergy) and time constraints.
On this page:
Images:
Indications
Suspected liver lesions such as hepatocellular carcinoma, focal nodular hyperplasia, adenomas, haemangiomas. Combined with the presence of scar tissue, or an injection of drug-eluting beads such as Lipiodol, whereby the unenhanced phase will act as a suitable baseline for comparing other phases.
Purpose
Differentiating liver lesions on non-contrast studies is difficult due to the homogeneity of the liver tissue on CT however this exam helps solve that problem. The portal vein accounts for~75% of the liver's blood supply with the remainder from the hepatic artery, so a later arterial phase is required for the best enhancement of the parenchyma.
To help characterise the vascularity of hypervascular liver lesions. This examination is most typically utilised to differentiate a hepatocellular carcinoma (HCC) from other lesions.
A hepatocellular carcinoma, a highly vascular primary lesion, will typically demonstrate hyperenhancement in the arterial phase and venous or delayed phase washout whilst a haemangioma should match the blood pool in each phase (same as the aorta in arterial etc.).
Technique
-
patient position
- supine with their arms above their head
-
scout
- diaphragm to iliac crests
-
scan extent
- diaphragm to iliac crests
-
scan direction
- craniocaudal
-
non-contrast phase
- no delay, performed before injection
-
contrast injection considerations (bolus tracking)
- monitoring slice (region of interest)
- level of the diaphragmatic hiatus or first lumbar vertebra at the aorta
- monitoring slice (region of interest)
-
threshold
- 150 HU
-
volume
- 100-120 mL of non-ionic contrast at 3 to 5 mL/s (a higher flow rate will equal greater enhancement 2)
-
scan delay 1
-
late arterial phase
- 15-30 seconds post bolus trigger (35-45 s after injection)
-
portal venous phase
- 60-75 seconds post-injection (independent of arterial timing)
-
delayed phase
- 2-5 minutes
-
late arterial phase
- respiration phase
- inspiration, breath-hold
Practical points
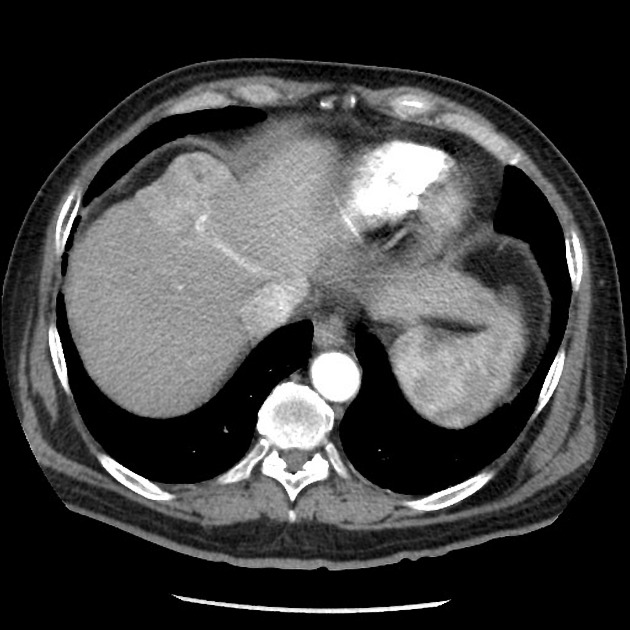
- an understanding of how each phase should appear is important 1:
- late arterial phase
- portal vein enhanced without hepatic venous enhancement
- hepatocellular carcinoma may only show enhancement in the late arterial phase so this is very important
- portal vein enhanced without hepatic venous enhancement
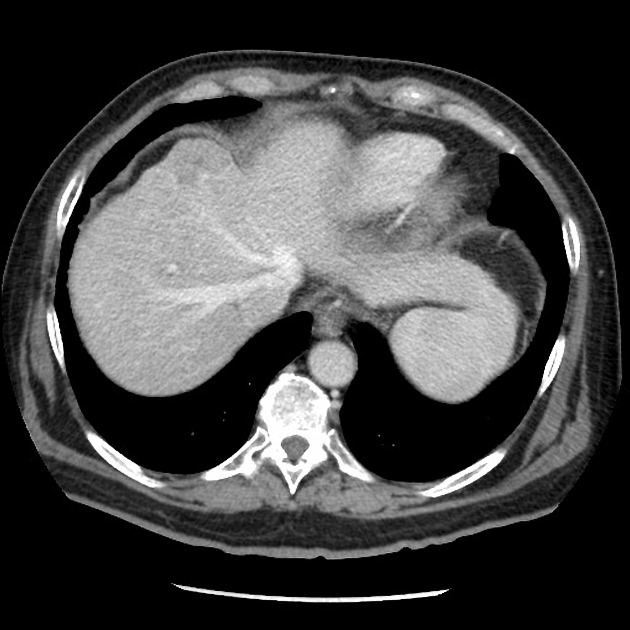
- portal venous phase
- portal veins, hepatic veins (via antegrade flow) enhanced
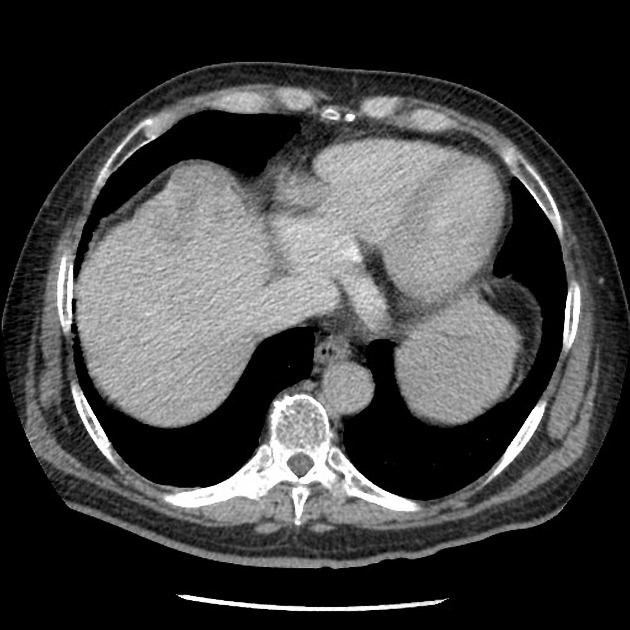
- delayed phase
- portal and hepatic veins slightly enhanced by a lot less than that portal venous phase
- late arterial phase
- slice thickness less than or equal to 5 mm





 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.