Neonatal hypoxic-ischaemic encephalopathy (HIE) is the result of a global hypoxic-ischaemic brain injury in a term neonate, usually after asphyxia.
On this page:
Terminology
It is important to remember that neonatal encephalopathy may result from a variety of conditions and hypoxic-ischaemic brain injury is the most important of them 1. Consequently, both terms are frequently used as synonyms.
Epidemiology
Hypoxic-ischaemic encephalopathy is one of the most common causes of cerebral palsy and other severe neurological deficits in children, occurring in 2-9 of every 1000 live births.
Clinical presentation
The encephalopathic neonate may have low Apgar scores at delivery and metabolic acidosis documented in the cord blood. Within the first 24 hours of life, the infant may develop symptoms of apnoea and seizures with abnormal electroencephalographic (EEG) results.
Pathology
The lack of sufficient blood flow, in conjunction with decreased oxygen content in the blood (perinatal asphyxia), leads to loss of normal cerebral autoregulation and diffuse brain injury.
The pattern of cerebral damage that results from perinatal/neonatal hypoxic-ischaemia depends on both gestational age (preterm or term) as well as the degree of insult (acute profound versus sustained partial asphyxia) 4. See patterns of neonatal hypoxic–ischaemic brain injury.
In general, pre-term infants with sustained partial asphyxia generally develop periventricular leukomalacia 4. Term infants with sustained partial asphyxia generally develop border zone ischaemia typically between the territories of the posterior cerebral artery and middle cerebral artery, commonly resulting in ulegyria 4. In contrast, term infants with profound asphyxia typically develop infarcts of the basal ganglia and areas of the cerebral cortex (e.g. precentral and postcentral gyri) 4.
The exact nature of the injury depends on the severity of hypotension and the degree of brain maturation. In general, the myelinated areas are more metabolically active and express more glutamate receptors (NMDA receptors), which make them more vulnerable to HIE due to excitotoxicity.
Radiographic features
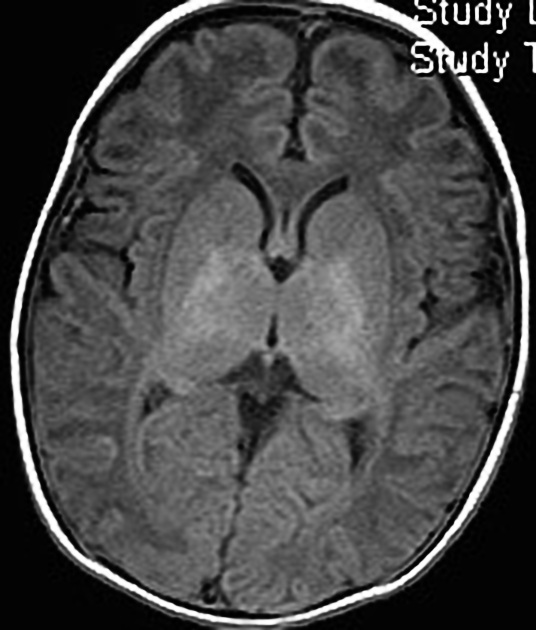
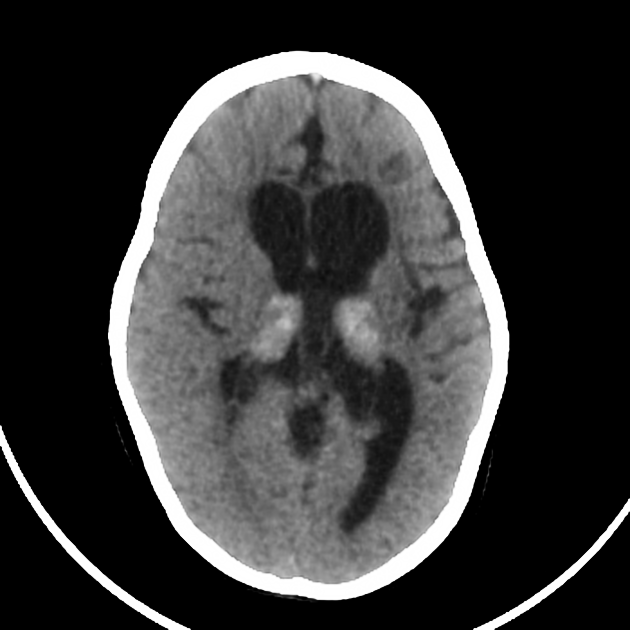
In term infants blood flow is ventriculofugal and changes are mainly, like in older children, in watershed-border zones; namely, parasagittal grey matter and subcortical white matter. Profound HIE in term babies results in thalamic and basal ganglia as well as sensorimotor cortex (perirolandic region) injury.
Ultrasound
Sonography is sensitive for the detection of haemorrhage, periventricular leukomalacia, and hydrocephalus. Resistive index (RI) of the middle cerebral arteries, if correlated with gestational age, can add more information. Severe HIE results in loss of autoregulation and increased RI.
CT
CT is the least sensitive modality for evaluation of HIE because of poor parenchymal contrast resolution in the neonatal brain due to the high water content of the parenchyma and high protein content of the CSF.
MRI
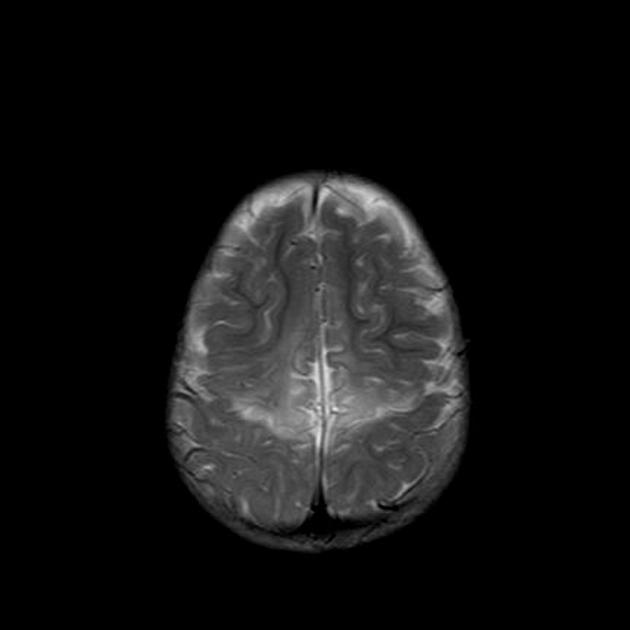
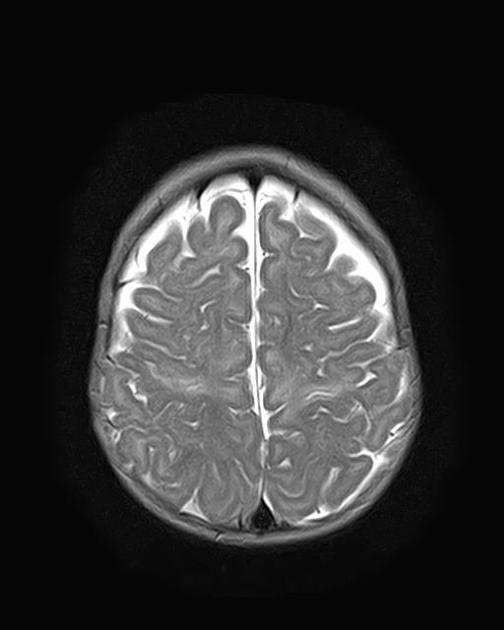
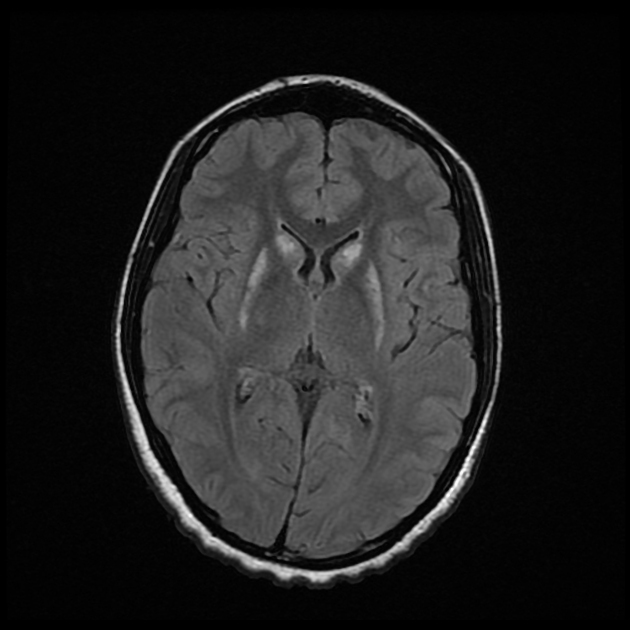
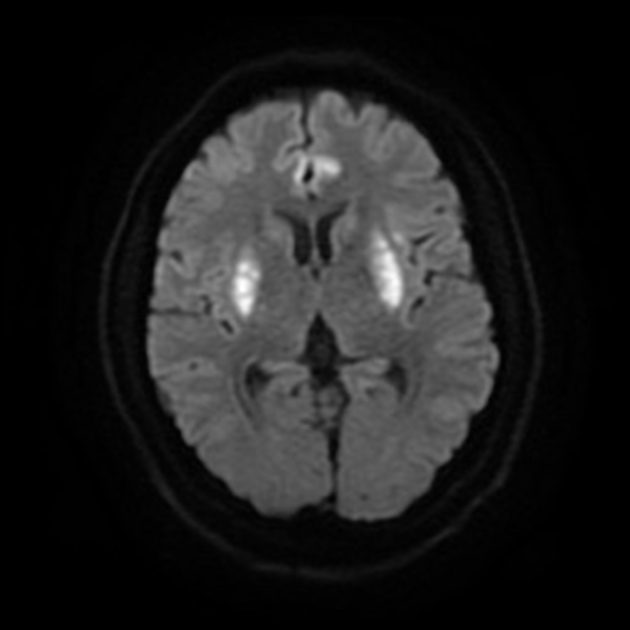
MRI is the most sensitive and specific imaging technique for examining infants with suspected hypoxic-ischaemic brain injury. Conventional sequences can help exclude other causes of encephalopathy such as haemorrhage, cerebral infarction, neoplasms, or congenital malformations.
A number of patterns of injury are encountered (see patterns of neonatal hypoxic-ischaemic brain injury) depending on the stage of brain maturation and severity of asphyxia, with the following expected signal intensity changes:
-
T1
grey matter: hyperintense
white matter: hypointense
-
T2
grey matter: variable depending on the time of imaging and presence of haemorrhage
white matter: hyperintense
-
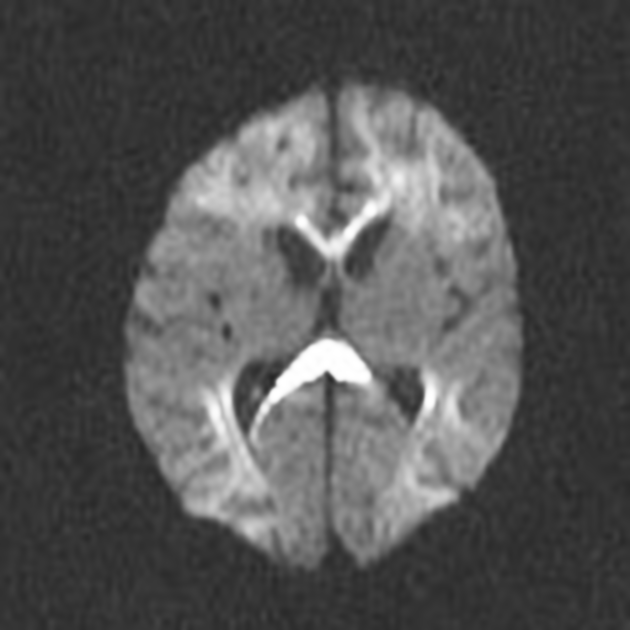
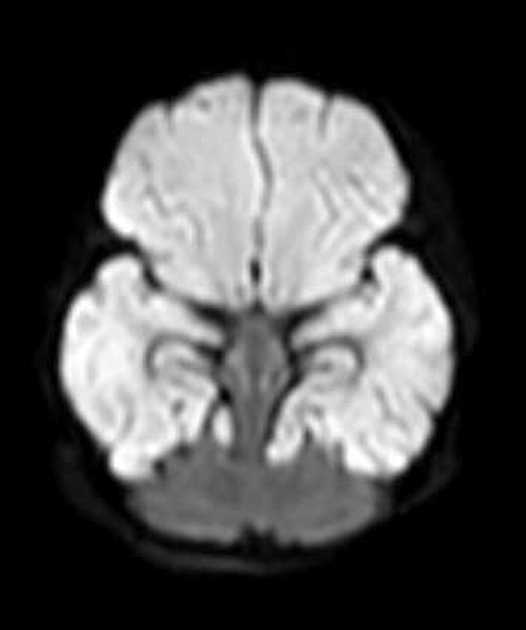
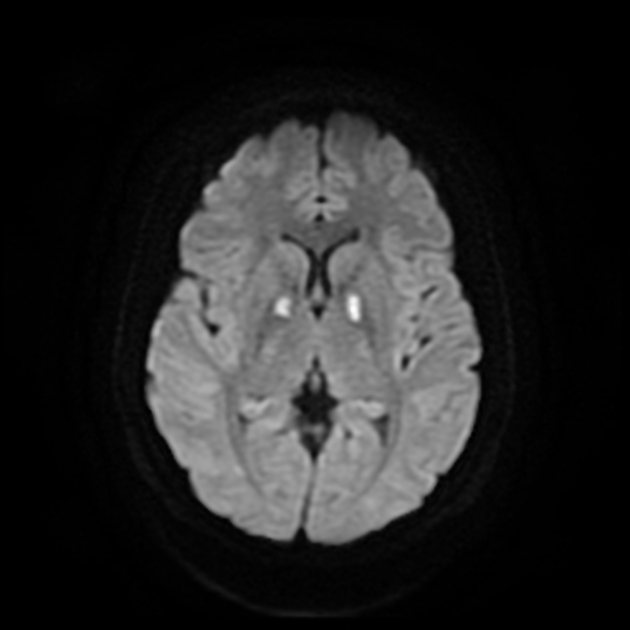
DWI/ADC
diffusion restriction first week
ADC pseudonormalisation occurs at the end of the first week
Treatment and prognosis
Increased severity of encephalopathy is indicated by the presence of cortical and basal ganglia abnormalities on conventional MR images, on diffusion-weighted MR images, and at MR spectroscopy. Severe EEG abnormalities also portend a poor outcome.
Although term infants with mild encephalopathy generally make a full recovery, 20% of affected infants die in the neonatal period and another 25% develop significant neurologic sequelae. For preterm infants, compared with term infants, the overall prognosis is worse.
Studies estimate a short therapeutic window of 2-6 hours during which interventions may be efficacious in reducing the severity of ultimate brain injury; thus, early identification of a neonate who has sustained a hypoxic-ischaemic insult is a paramount objective for optimal management and treatment.





 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.