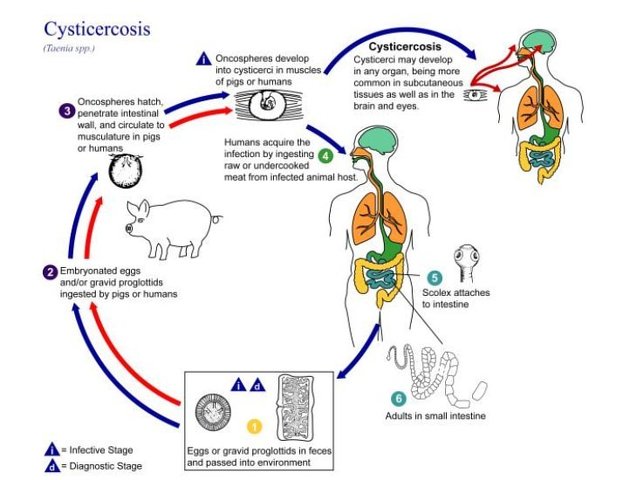
Neurocysticercosis is caused by the CNS infection with the pork tapeworm Taenia solium, which is endemic in most low-income countries where pigs are raised. This form of cysticercosis is a relevant cause of seizures in endemic areas.
On this page:
Epidemiology
The disease is endemic in Central and South America, Asia and Africa. The perpetuation of this parasitic disease is related to poor sanitation and hygiene.
There is no gender or race predilection and most symptomatic patients are aged 15-40 years 4.
Clinical presentation
There is a variable time interval between the point of infection and the onset of symptoms (ranging from 1-30 years).
Clinical presentation includes 1:
seizures: most common symptom and the most common cause of seizures in young adults in endemic areas 2
headaches
altered mental status
neurological deficits
Bruns syndrome: caused by cysticerci cysts of the third and fourth ventricle 4
CSF serology may be helpful with the initial diagnosis, especially in cases of intraventricular/subarachnoid infection 2.
Pathology
Infection, which leads to extraintestinal disease (including neurocysticercosis), usually occurs as a result of eating food or drinking water contaminated by human feces containing T. solium eggs. This is distinct from the 'normal' life cycle in which the undercooked pork is eaten and the larval cysts contained within, mature into adult intestinal tapeworm 3.
Extra-intestinal infection undergoes specific clinical and imaging changes at it progresses through four stages of infection 1.
Stages
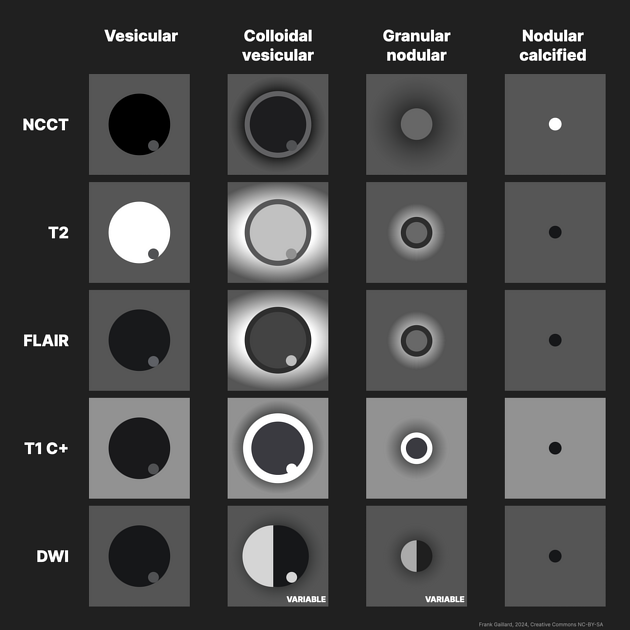
There are four main stages (also known as Escobar's pathological stages):
vesicular: viable parasite with intact membrane and therefore no host reaction.
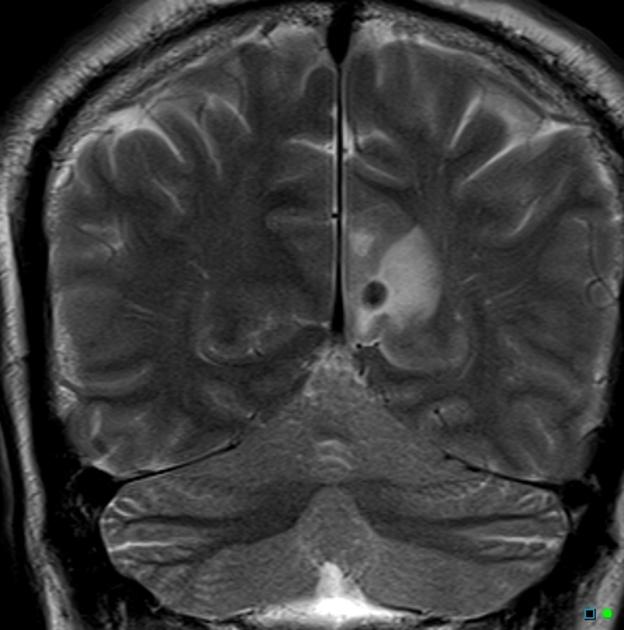
colloidal vesicular: parasite dies within 4-5 years 1 untreated, or earlier with treatment and the cyst fluid becomes turbid. As the membrane becomes leaky edema surrounds the cyst. This is the most symptomatic stage.
granular nodular: edema decreases as the cyst retracts further; enhancement persists.
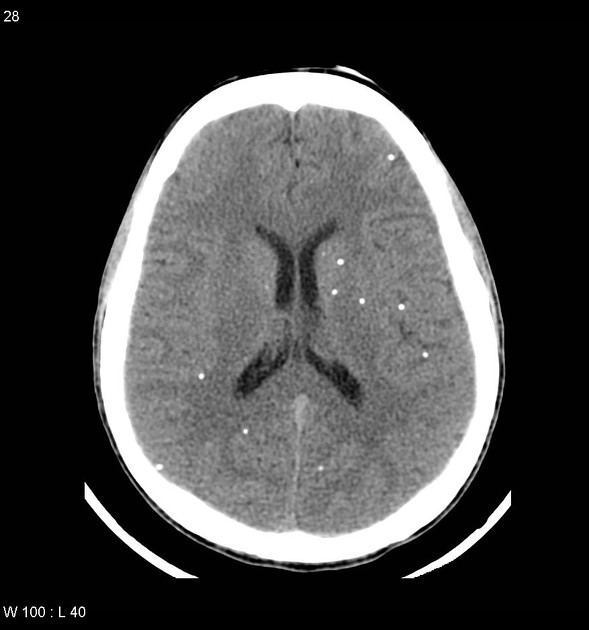
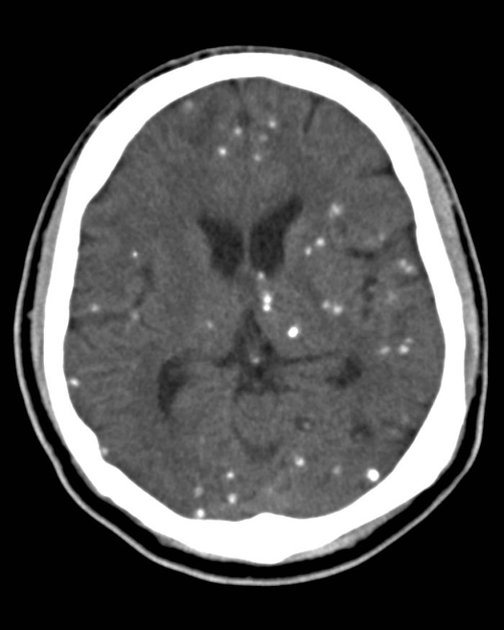
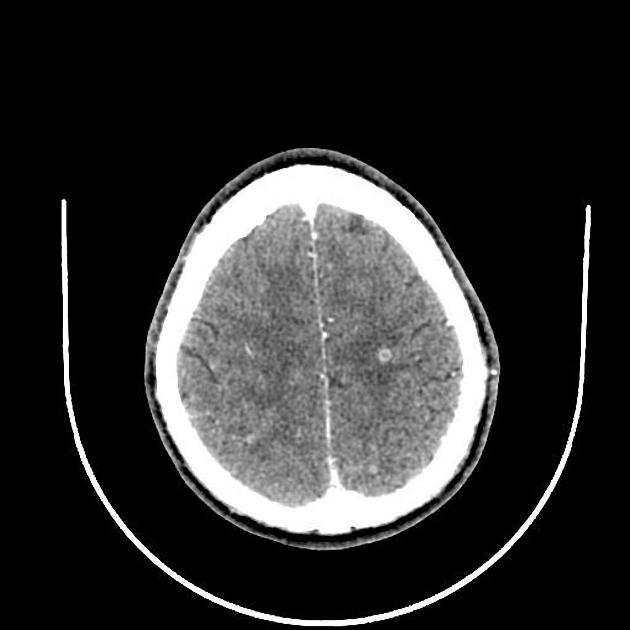
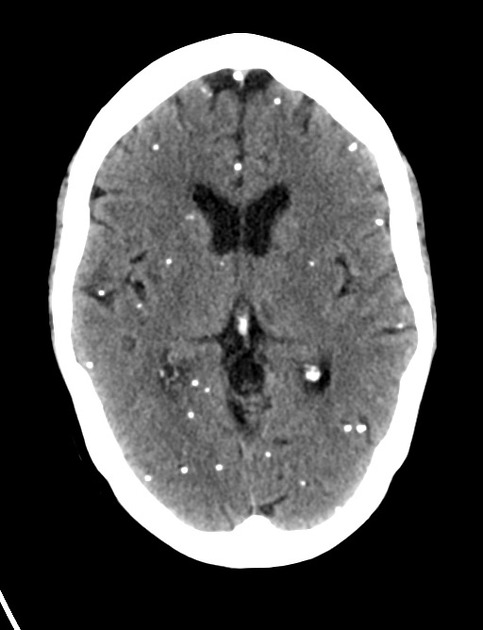
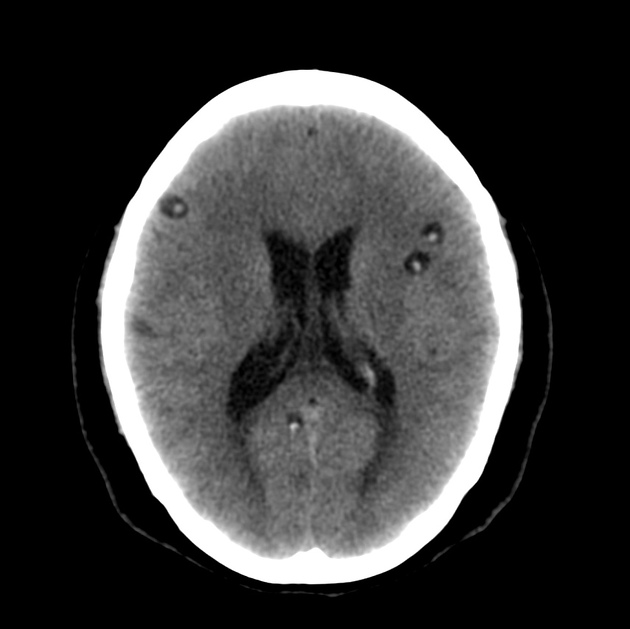
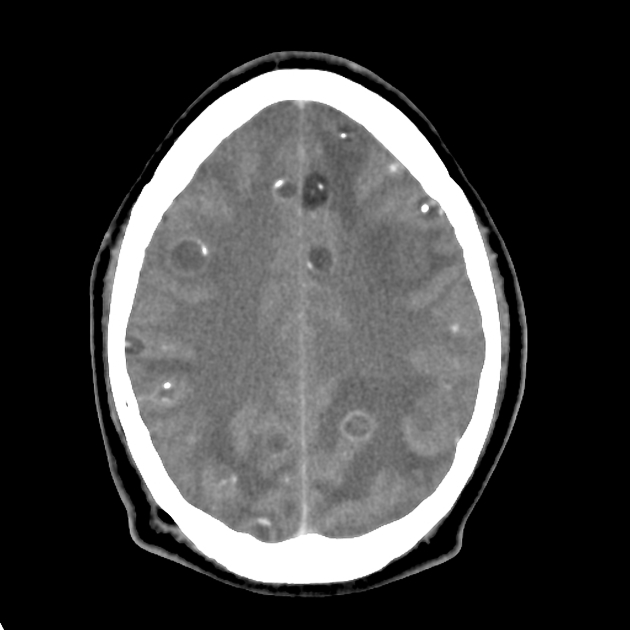
nodular calcified: end-stage quiescent calcified cyst remnant; no edema.
Radiographic features
Imaging findings depend on the location and stage of infection.
Location
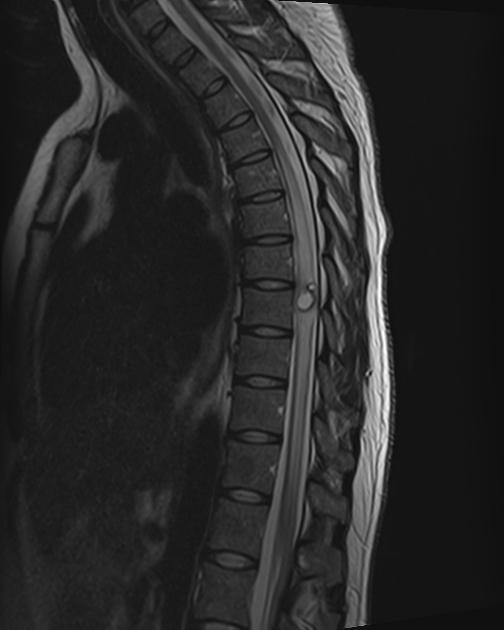
Cysts can occur essentially anywhere in the neuraxis and can be both intraaxial or extraaxial 3-5.
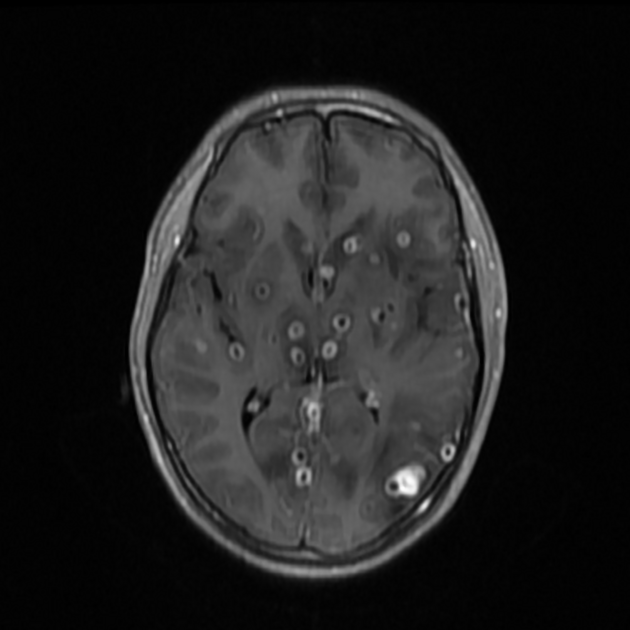
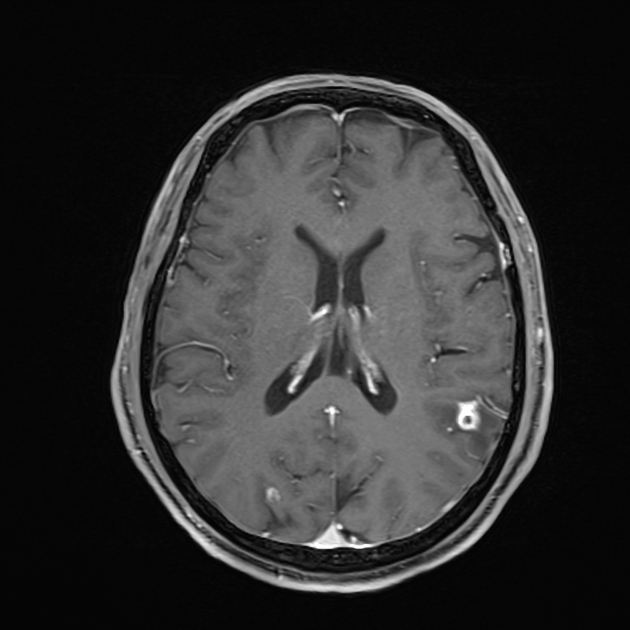
parenchyma: most common
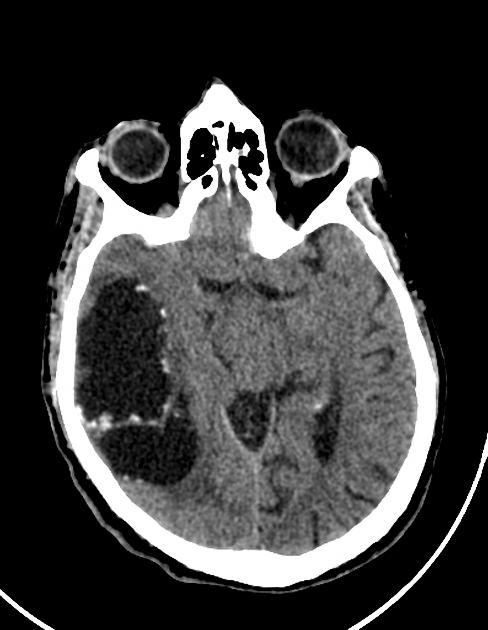
subarachnoid space over the cerebral hemispheres: can be very large
ventricles
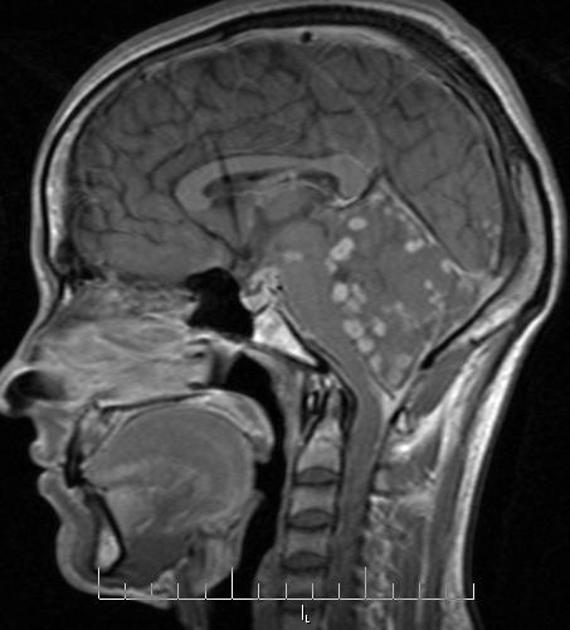
spinal forms: usually associated with concomitant intracranial involvement 4
Parenchymal
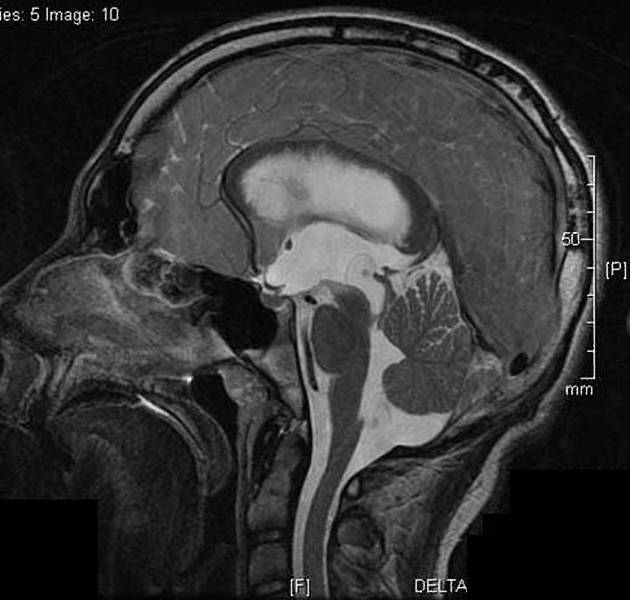
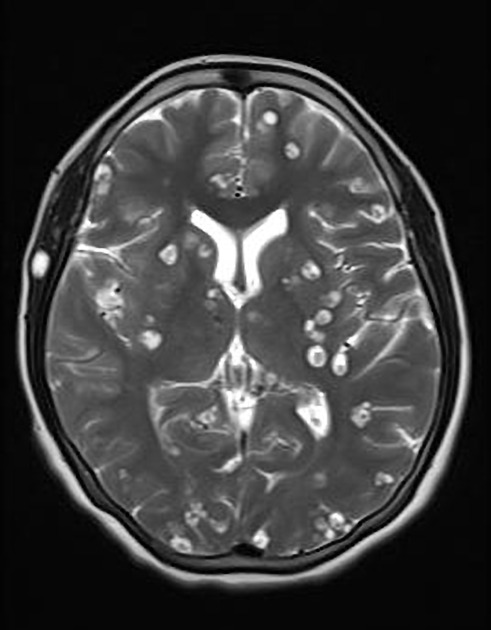
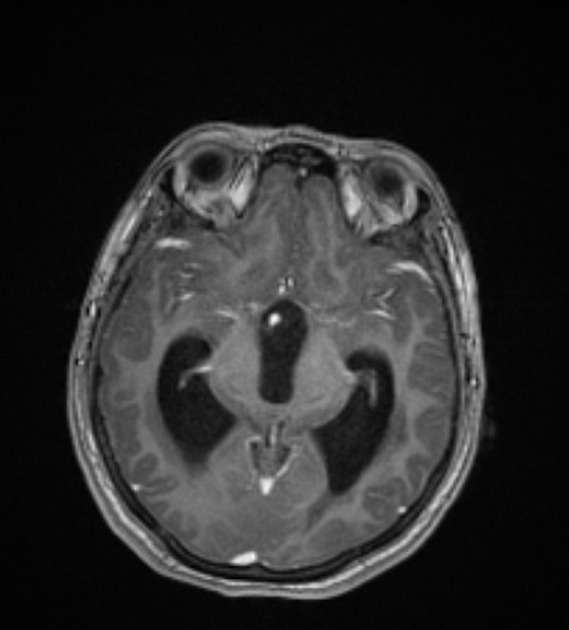
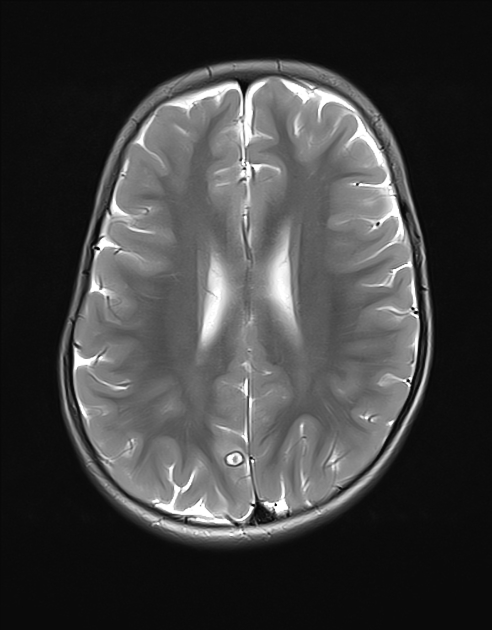
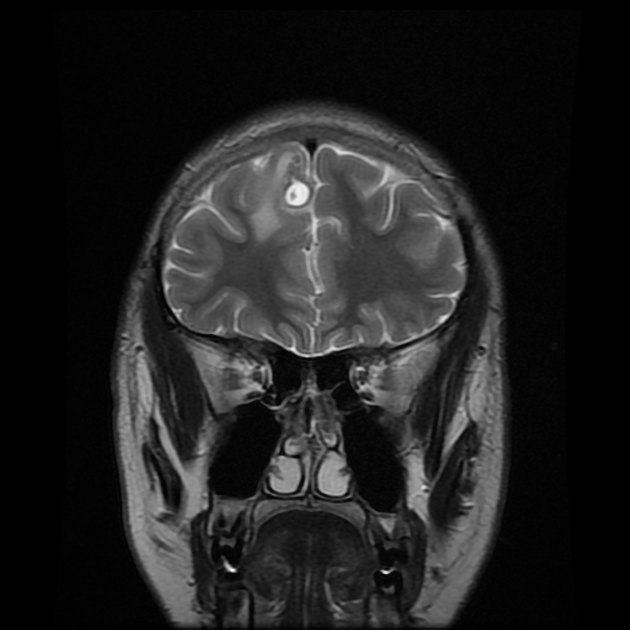
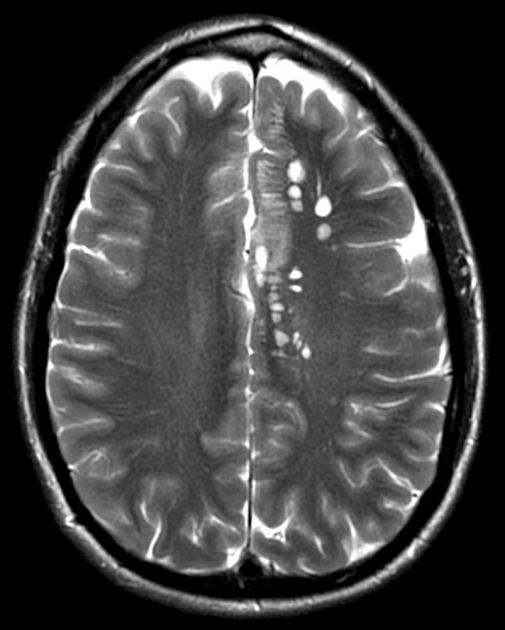
Parenchymal cysts usually involve the grey-white matter junction 2,4. They are usually small in size ~10mm.
Subarachnoid/intraventricular
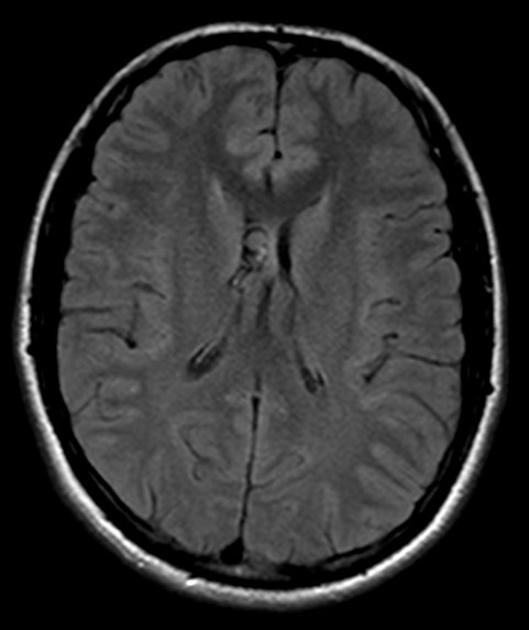
When in the subarachnoid space or within the ventricles, the cysts typically do not have a visible scolex. Usually, the cysts are similar in signal intensity to CSF, although occasionally cyst fluid may somewhat differ 2. They may have peripheral calcification.
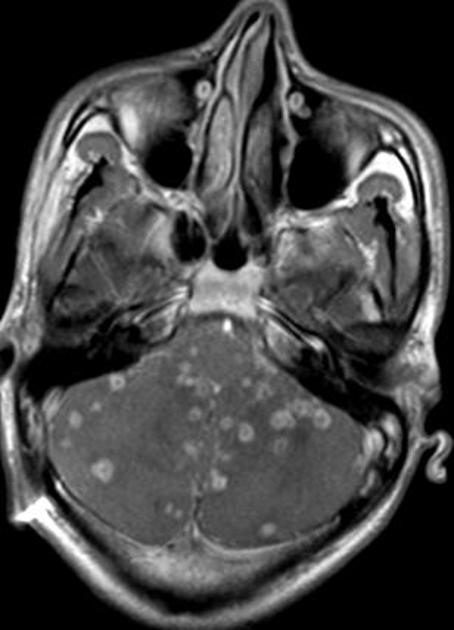
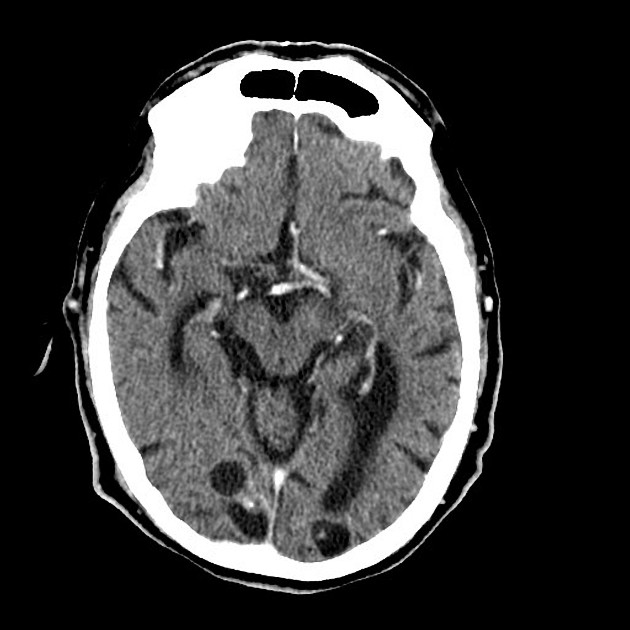
In the basal cisterns, they can be clustered grape-like (racemose neurocysticercosis). The cysts are typically 1-2 cm in diameter 2.
Elsewhere in the subarachnoid space, particularly in the sylvian fissure, cysts can be much bigger (up to 9 cm) 7.
In the ventricles, there is often (79%) 2 associated ventriculitis often leading to aqueductal stenosis and hydrocephalus 2.
Stage
Each stage has fairly distinctive imaging features although there is no sharp demarcation between later stages.
Vesicular
fluid is CSF density (CT)/intensity (MRI)
-
eccentric scolex can sometimes be seen 2,6,8
high signal compared to fluid on T1, DWI, FLAIR
low signal compared to fluid on T2, ADC
no enhancement is typical, although very faint enhancement of the wall and enhancement of the scolex may be seen 6
no surrounding vasogenic edema
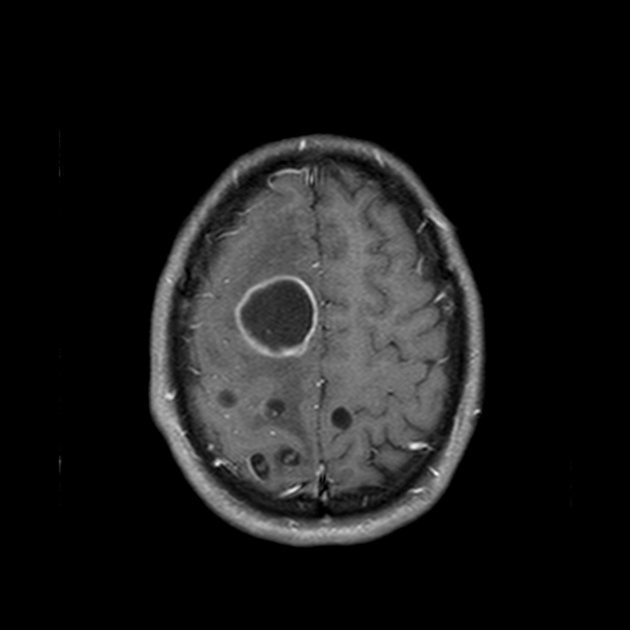
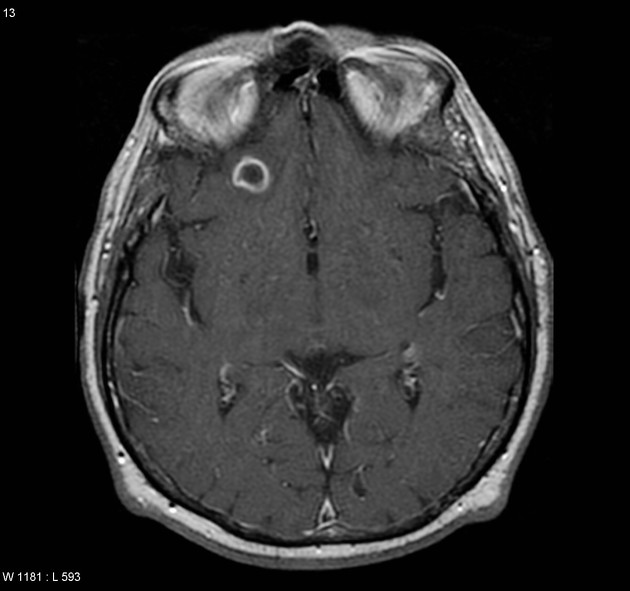
Colloidal vesicular
-
cyst fluid becomes macroscopically turbid reflected in altered imaging appearance:
CT: hyperattenuating to CSF
-
MRI
T1, FLAIR hyperintense to CSF 2,8
DWI/ADC is variable, ranging from similar to CSF to frank diffusion restriction (high DWI signal, low ADC values) 8
surrounding edema
cyst and the wall become thickened and brightly enhances
scolex is seen early in the colloidal phase, similar to vesicular stage, but gradually shrinks down and becomes harder to identify 6
Granular nodular
edema decreases
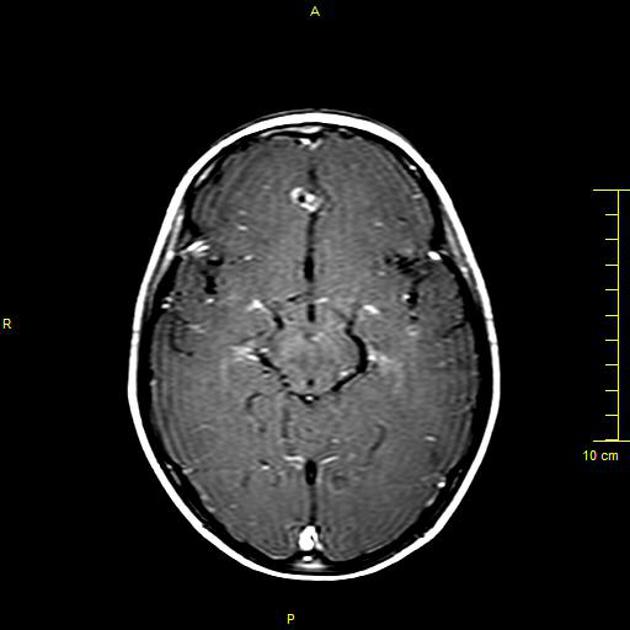
cyst gradually retracts eventually becoming a small enhancing nodule 6
cyst fluid may demonstrate diffusion restriction in early granular nodular stage, disappearing during late phase as calfication occurs 8
enhancement persists but is less marked 1
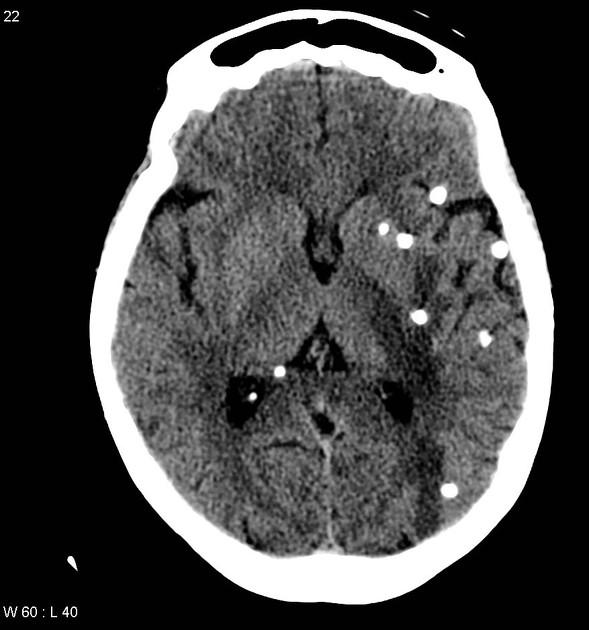
Nodular calcified
end-stage quiescent calcified nodule
no edema
no enhancement on CT
signal drop out on T2 and T2* sequences
some intrinsic high T1 signal may be present
long term enhancement may be evident on MRI and may predict ongoing seizures 1
A helpful mnemonic to remember these stages is Vegans Can't Get Neurocysticercosis.
Treatment and prognosis
The treatment options available to patients with neurocysticercosis include symptomatic therapy (e.g. antiseizure medications) and anthelmintic therapy (e.g. albendazole and praziquantel, the two antiparasitics most commonly used 4), usually accompanied by corticosteroids. Surgery (e.g. VP shunt placement or decompression) is only rarely indicated.
Differential diagnosis
General imaging differential considerations include:
pyogenic cerebral abscess
other parasitic/fungal infection(s)
When large and in the subarachnoid space, they may mimic an arachnoid cyst or an epidermoid cyst.







 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.