Creutzfeldt-Jakob disease (CJD) is a transmissible spongiform encephalopathy that results in rapidly progressive dementia and death usually within a year from onset. The vast majority are sporadic, but familial and acquired forms are occasionally encountered.
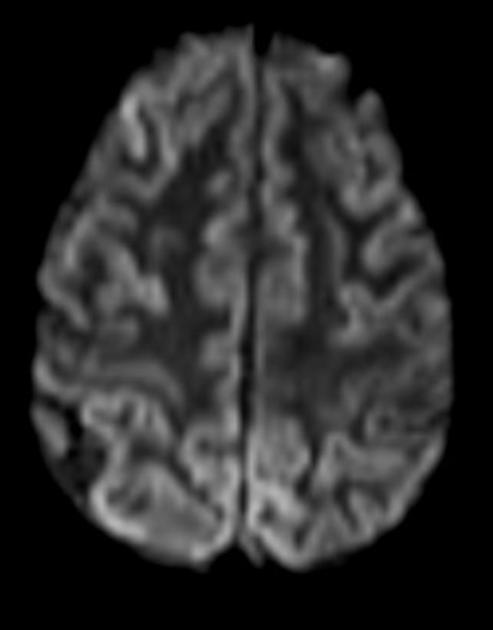
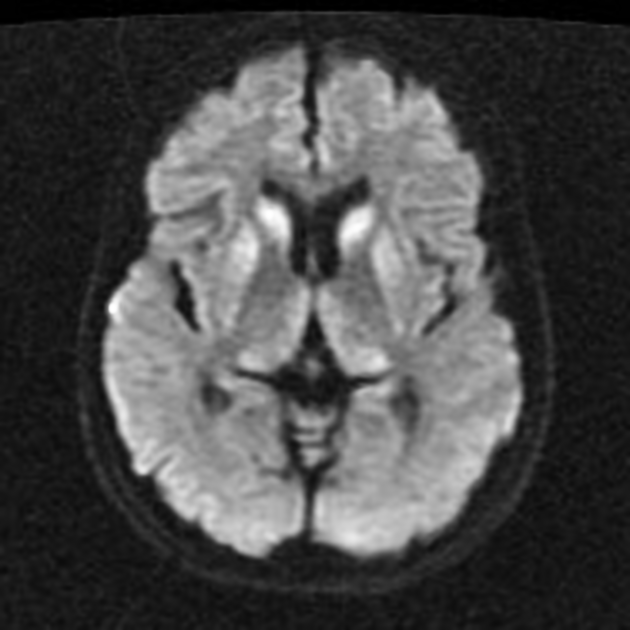
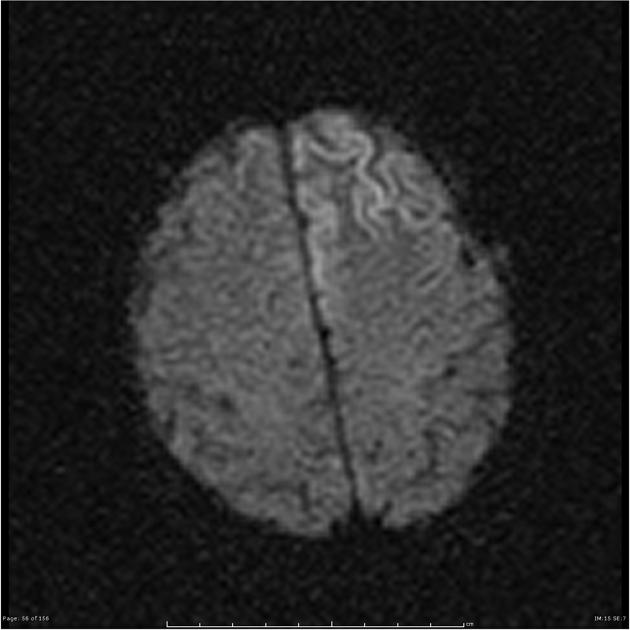
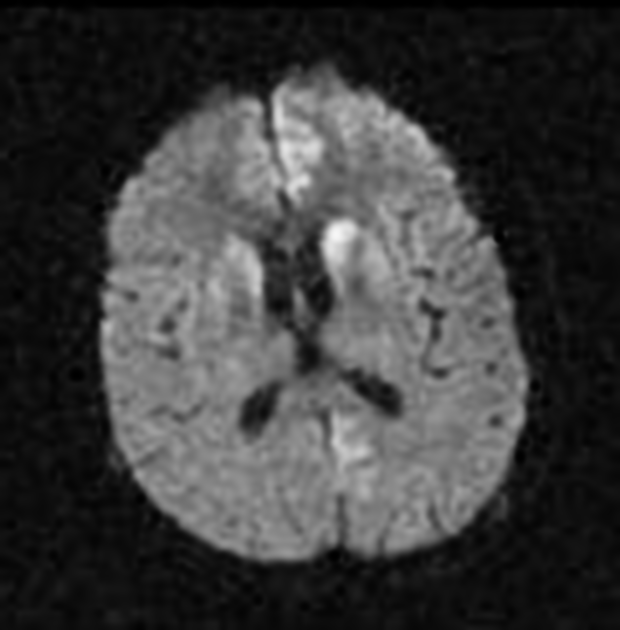
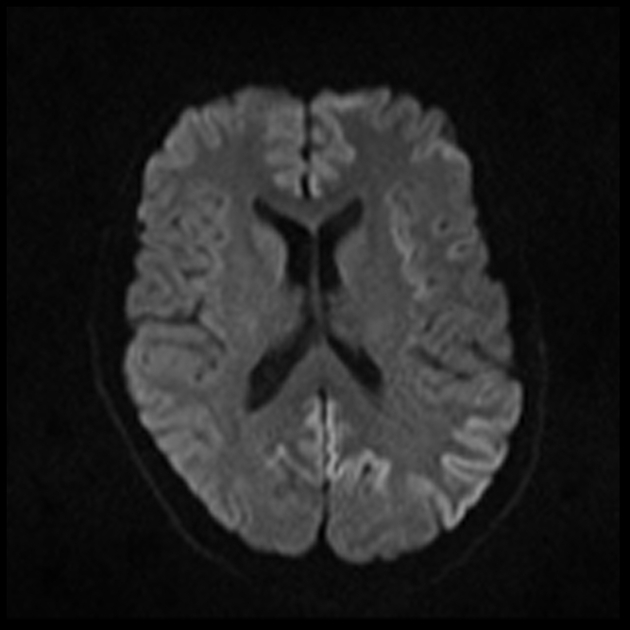
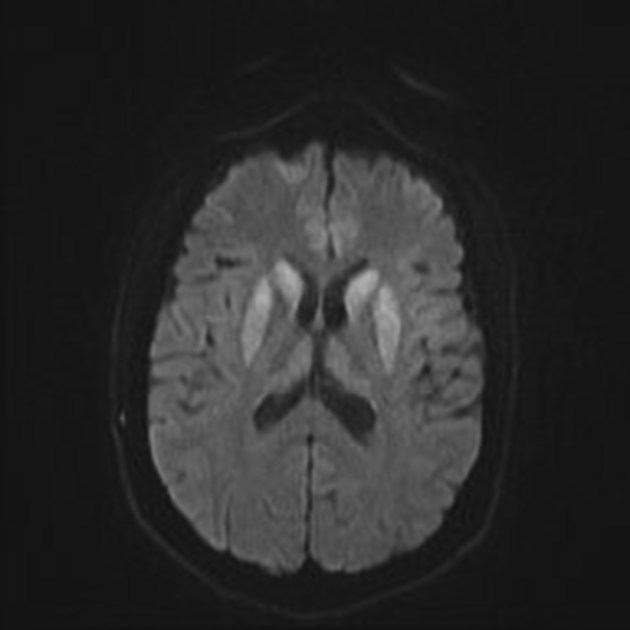
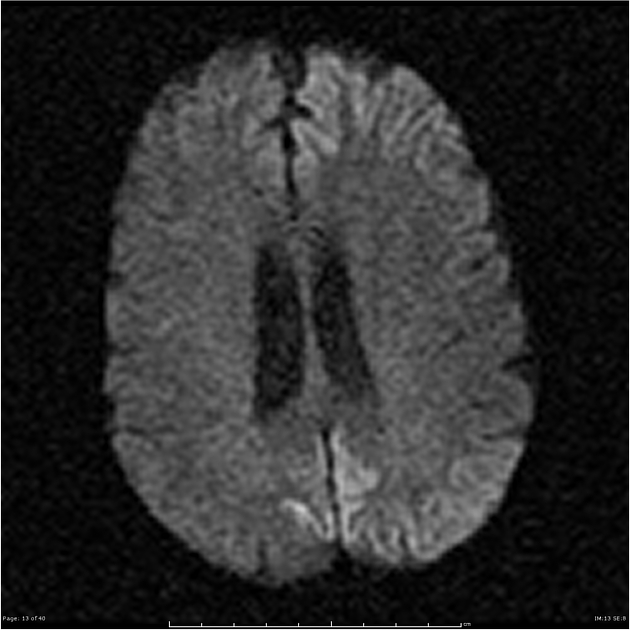
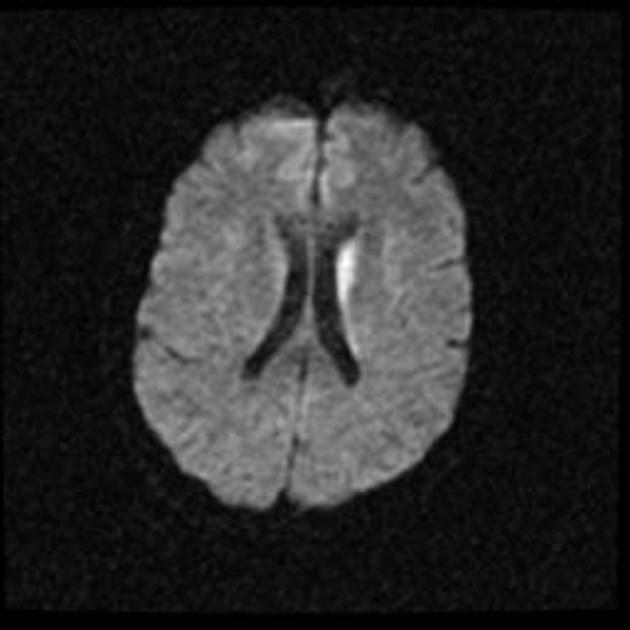
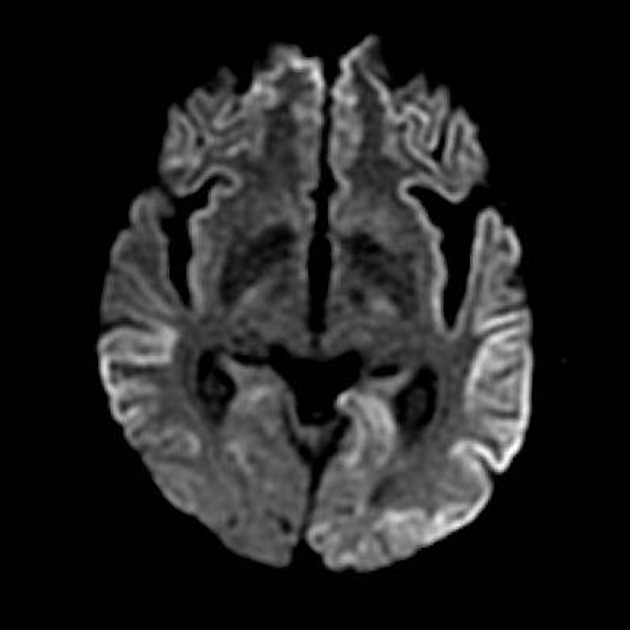
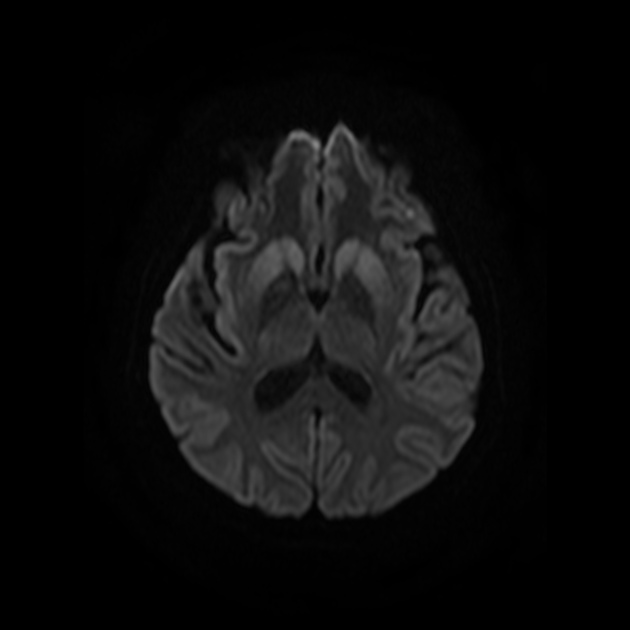
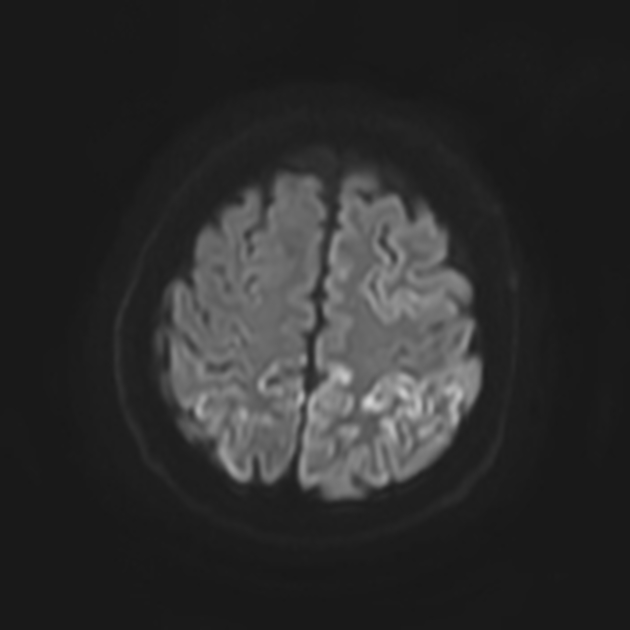
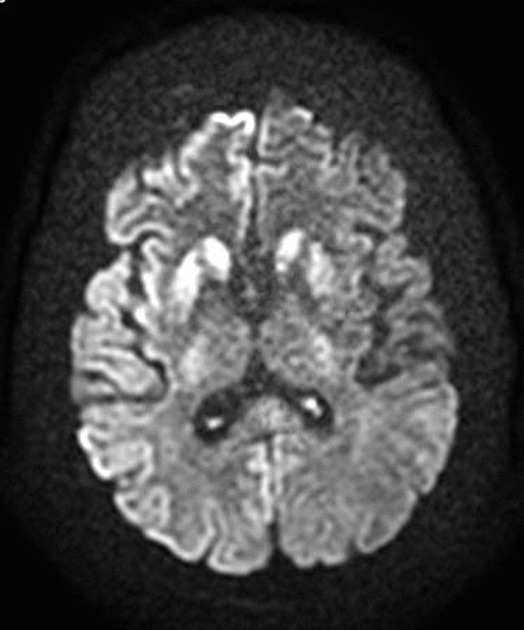
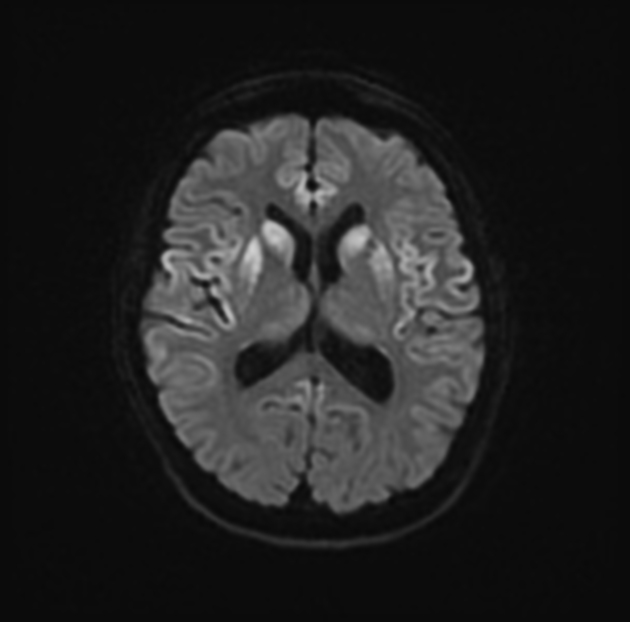
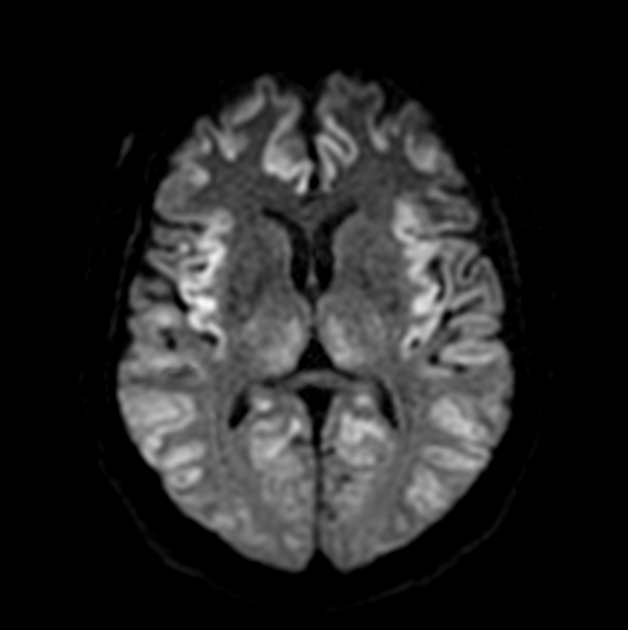
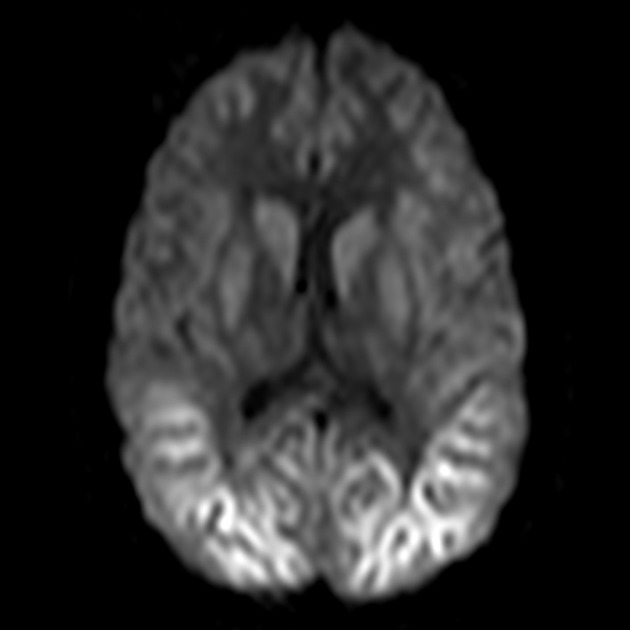
On imaging, it classically manifests as hyperintense signal on DWI (and usually FLAIR) in regions of the cerebral grey matter (cortex, followed by the striatum, followed by thalamus).
On this page:
Epidemiology
Four types of Creutzfeldt-Jakob disease have been described 2,6:
-
sporadic (sCJD)
accounts for 85-90% of cases
further divided into numerous subtypes according to molecular markers (see pathology section below)
-
variant (vCJD)
bovine-to-human transmission of bovine spongiform encephalopathy (a.k.a. "mad cow disease"): considered zoonotic by some
especially if in the UK between 1981-1996
subsequent human-to-human transmission (e.g. transfusion) of vCJD
-
familial (fCJD)
10% of cases
these individuals carry a PRPc mutation
-
iatrogenic (iCJD)
following administration of cadaveric human pituitary hormones (pre-1985)
various transplants and other procedures (e.g. cadaveric dural grafting)
Diagnosis
The United State of America's Centres for Disease Control and Prevention (CDC) defines the following diagnostic criteria 27:
-
sporadic Creutzfeldt-Jakob disease (sCJD)
definite: diagnosed by standard neuropathological and/or immunocytochemically and/or Western blot confirmed protease-resistant PrP and/or presence of scrapie-associated fibrils
-
probable
neuropsychiatric disorder plus positive RT-QuIC in CSF or other tissues
orrapidly progressive dementia; and at least two out of the following four clinical features: myoclonus; visual or cerebellar signs; pyramidal/extrapyramidal signs; akinetic mutism AND a positive result on at least one of the following laboratory tests: typical EEG; positive 14-3-3 CSF assay with disease duration <2 years; DWI or FLAIR high signal in the caudate/putamen or at least cortical regions (temporal, parietal, occipital) AND without routine investigations indicating an alternative diagnosis
possible: progressive dementia; and at least two out of the four clinical features above AND the absence of a positive result for any of the four tests above AND duration of illness <2 years
AND without routine investigations indicating an alternative diagnosis
iatrogenic Creutzfeldt-Jakob disease (iCJD): progressive cerebellar syndrome in a recipient of human cadaveric-derived pituitary hormone; or sporadic Creutzfeldt-Jakob disease with a recognised exposure risk, e.g. antecedent neurosurgery with dura mater implantation
familial Creutzfeldt-Jakob disease (fCJD): definite or probable Creutzfeldt-Jakob disease plus definite or probable Creutzfeldt-Jakob disease in a first degree relative; and/or neuropsychiatric disorder plus disease-specific PrP gene mutation
Clinical presentation
Sporadic Creutzfeldt-Jakob disease
Sporadic Creutzfeldt-Jakob disease is characterised by rapidly progressive dementia and other features of neuropsychiatric decline resulting in death within a year of onset. Other common central features include myoclonus, visual hallucinations, cerebellar dysfunction (such as ataxia and nystagmus), pyramidal or extrapyramidal signs (such as spasticity, rigidity, dystonia, or bradykinesia), and eventually akinetic mutism 22. Peripheral nervous system involvement can be seen in 10% of cases 31.
Phenotypic variants 22,28:
amyotrophic variant: initial amyotrophic lateral sclerosis-like presentation
Brownell-Oppenheimer variant: initial cerebellar ataxia
Heidenhain variant: initial visual symptoms such as impaired visual acuity, distortions of shapes and colours, and visual hallucinations
Stern-Garcin variant: initial extrapyramidal features
Variant Creutzfeldt-Jakob disease
Variant Creutzfeldt-Jakob disease presents mostly with psychiatric symptoms (such as depression) and sensory symptoms (dysaesthesias or paraesthesias).
Diagnostic markers
characteristic results on electroencephalography (EEG)
CSF 14-3-3 protein: positive result in a patient suspected clinically of having sporadic Creutzfeldt-Jakob disease
-
CSF and/or olfactory mucosa real-time quaking-induced conversion (RT-QuIC) seeding assays: detects minute amounts of the disease-specific pathologic prion protein 12,13
found to be more sensitive than CSF 14-3-3 protein titres 12
A definitive diagnosis requires a brain biopsy, although in many institutions the difficulty involved in sterilising equipment renders a biopsy undesirable.
Pathology
Creutzfeldt-Jakob disease is mediated via prions, a type of protein, which manifests in sheep as the disease scrapie, and in cows as bovine spongiform encephalopathy. Prions are considered infectious in the sense that they can alter the structure of neighbouring proteins.
Creutzfeldt-Jakob disease leads to spongiform degeneration of the brain, which is thought to be caused by the conversion of normal prion protein to proteinaceous infectious particles that accumulate in and around neurones and lead to cell death.
Classification
A number of subtypes of sporadic Creutzfeldt-Jakob disease are recognised based on molecular markers, and these have distinct clinical and pathological features. The classification is based on 16,17:
-
codon 129 in the prion protein gene
methionine (M) or valine (V)
-
size of the protease-resistant core of the abnormal prion protein
PrPSc type 1 (21 kDa) or type 2 (19 kDa)
Each variant of sporadic Creutzfeldt-Jakob disease results from the combination of codon 129 genotype (M or V) and PrPSc type (1, 2 or 1 + 2) 16,17.
Additionally, the MM2 subtype has been noted to have distinctive histopathological features affecting the cerebral cortex or the thalamus and have therefore been separated into MM2 cortical (MM2C) and MM2 thalamic (MM2T).
Mixed types are also encountered. The result is that there is significant variation in the literature in regards to how many subtypes there are and if and how they should be grouped together 17.
Clinical patterns have been linked to various molecular subtypes (e.g. Heidenhain variant linked to MM1 and MM2C 18, and Brownell-Oppenheimer variant linked to VV2 19,20).
Radiographic features
Specific sites of imaging abnormality are typical of sporadic Creutzfeldt-Jakob disease 22:
-
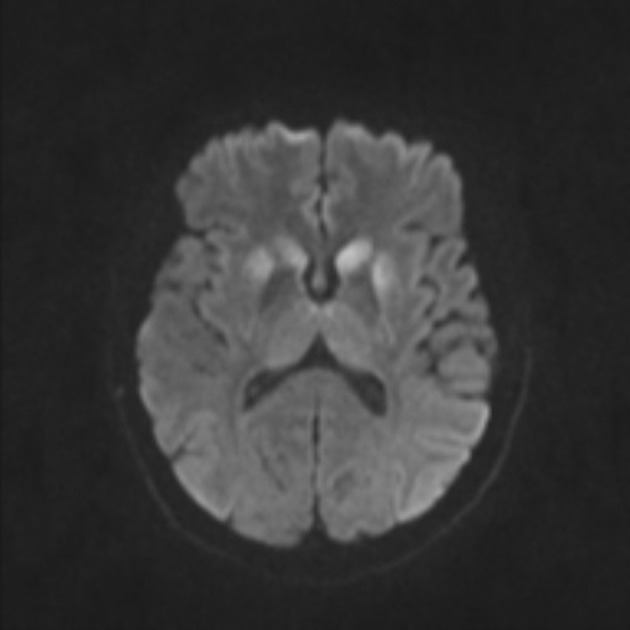
cerebral cortex (most common) 5,9:
most common: insula, cingulate gyrus, superior frontal gyrus
common: precuneus, cuneus, paracentral lobule, medial frontal gyrus, occipital gyri, angular/supramarginal gyrus, superior parietal lobule, inferior frontal gyrus
less common: postcentral gyrus, precentral gyrus, medial and superior temporal gyri
-
deep grey matter
Involvement is usually bilateral but may be asymmetric or symmetric 5. The extent of abnormality increases as the disease progresses.
Phenotypic variants of sporadic Creutzfeldt-Jakob disease also have characteristic initial radiographic features 22,23:
Brownell-Oppenheimer variant: initially involves the cerebellum and sometimes basal ganglia, and may not be visible until atrophy develops 22-23
Heidenhain variant: initially involves the parieto-occipital cortex
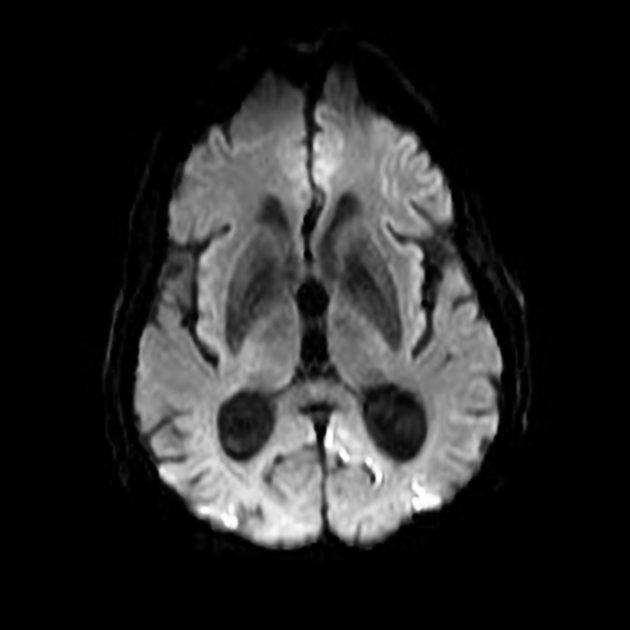
Stern-Garcin variant: initially involves the basal ganglia (striatum) and thalamus
Variant Creutzfeldt-Jakob disease characteristically shows the hockey stick sign and/or pulvinar sign of thalamic involvement. However, thalamic involvement is not pathognomonic of variant disease and is more commonly seen with sporadic Creutzfeldt-Jakob disease due to the overall greater prevalence of the sporadic Creutzfeldt-Jakob disease 22.
MRI
MRI is the modality of choice to assessing patients with suspected Creutzfeldt-Jakob disease.
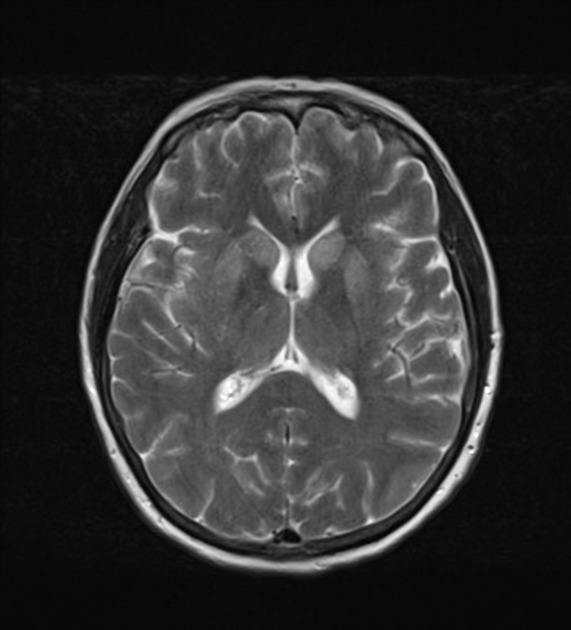
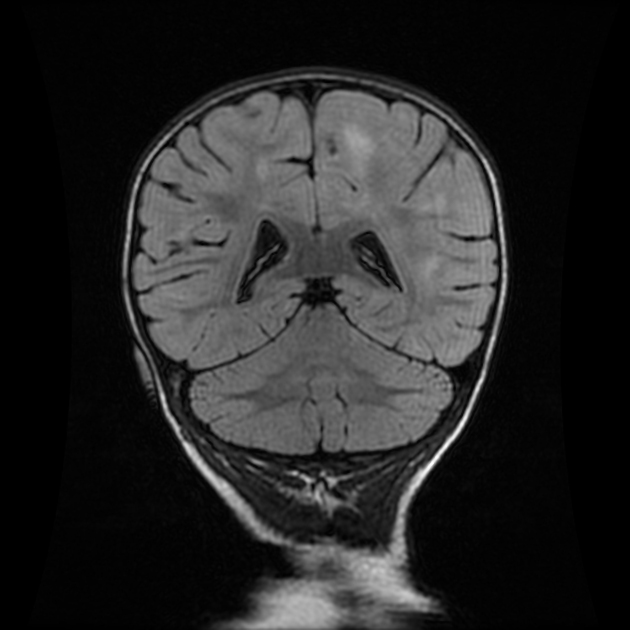
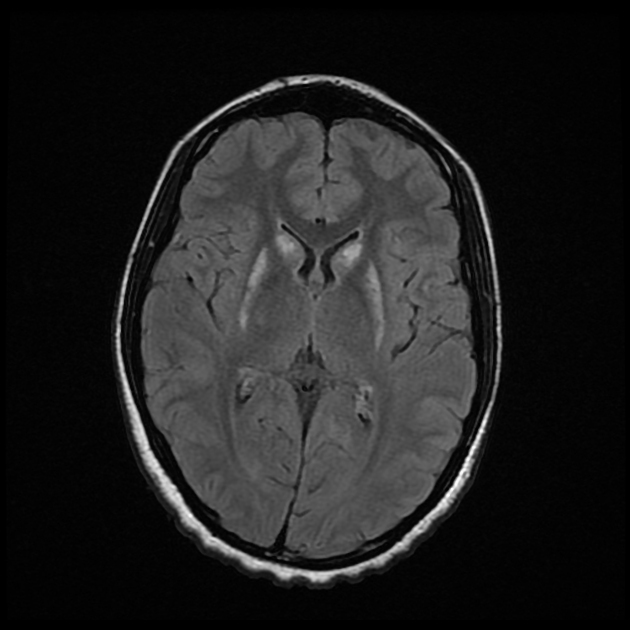
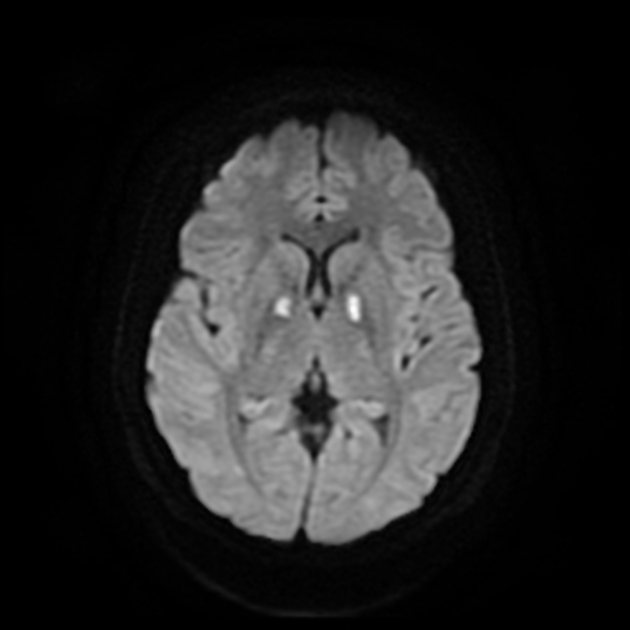
The most sensitive sequence to identify characteristic changes is diffusion-weighted imaging (e.g. b=1000) which demonstrates increased signal, that is more conspicuous than either T2/FLAIR changes and ADC abnormalities 8,22-24. Signal abnormalities may be subtle initially but become more pronounced as the disease progresses. Review of sequential studies also typically demonstrates rapidly progressive cerebral atrophy.
-
DWI: hyperintensity (most sensitive) and more pronounced than T2/FLAIR changes due to a combination of true diffusion restriction and so-called T2 shine through
including cortical ribboning, the hockey stick sign, and the pulvinar sign
-
ADC: variable, depends on timing
early: low values - these may be seen prior to marked changes on DWI or visible FLAIR changes
late: pseudonormalised or facilitated and associated with atrophy 22-24
T2/FLAIR: hyperintensity is more subtle than DWI changes and may be absent early in the course of the disease
T1: may show high signal in globus pallidus (uncommon) 25,30
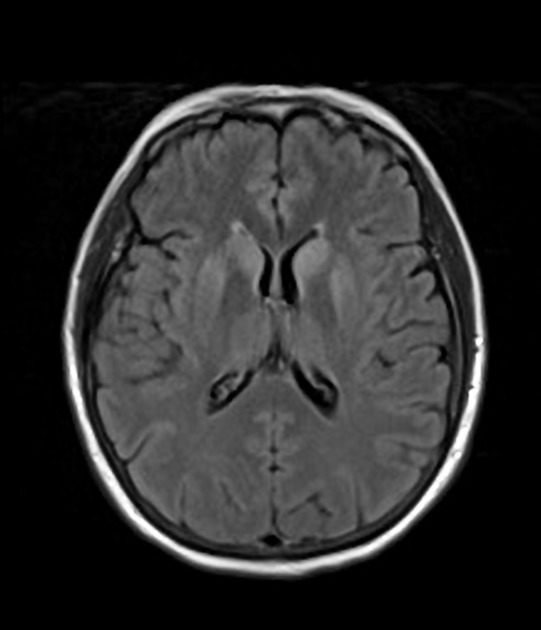
T1 C+ (Gd): no abnormal enhancement
Nuclear medicine
Fluorine-18-FDG PET shows hypometabolism in the affected regions 29.
Treatment and prognosis
There is no curative treatment and the disease is invariably fatal with a mean survival of seven months.
Creutzfeldt-Jakob disease is a notifiable disease in most countries, including European Union countries, Australia, United Kingdom, USA, and Canada.
History and etymology
It was named after Hans Gerhard Creutzfeldt (1885-1964), a German neurologist who first described the condition in 1920, and Alfons Maria Jakob (1884-1931), a German neurologist who also described the condition in a separate study in 1921 14,15.
Differential diagnosis
MRI imaging features overlap with many other conditions but the presentation will be different 8,10,11,21:
-
particularly anti-D2 dopamine antibodies
T2 signal change is dominant, with only minor, if any, diffusion change








 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.