Pancreatic ductal adenocarcinoma, frequently referred to as pancreatic cancer, makes up the vast majority (~90%) of all pancreatic neoplasms and remains a disease with a very poor prognosis and high morbidity.
On this page:
Epidemiology
Pancreatic cancer accounts for 22% of all deaths due to gastrointestinal malignancy, and 5% of all cancer deaths 1. In general, it is a malignancy of the elderly with over 80% of cases occurring after the age of 60 1.
Risk factors
Risk factors include:
cigarette smoking: the strongest environmental risk factor
a diet rich in animal fats and protein
family history: three or more first-order relatives with pancreatic cancer results in ~20x increase in risk 8
-
hereditary syndromes 6
BRCA2 mutations are the most frequent cause of familial pancreatic cancer 12
familial breast cancer
There is only a weak association, if at all, with heavy alcohol consumption alone, though chronic pancreatitis is a risk factor 1.
Clinical presentation
pain (most common)
Courvoisier gallbladder: painless jaundice and enlarged gallbladder
Trousseau syndrome: migratory thrombophlebitis
new-onset diabetes mellitus
-
lipase hypersecretion syndrome (10-15%) 9
polyarthralgia and subcutaneous fat necrosis +/- lytic bone lesions
elevated serum lipase and eosinophilia
Pathology
Three precursor lesions for pancreatic adenocarcinoma have been identified 8:
pancreatic intraepithelial neoplasia (PanIN): responsible for more than 90% of pancreatic cancers 12
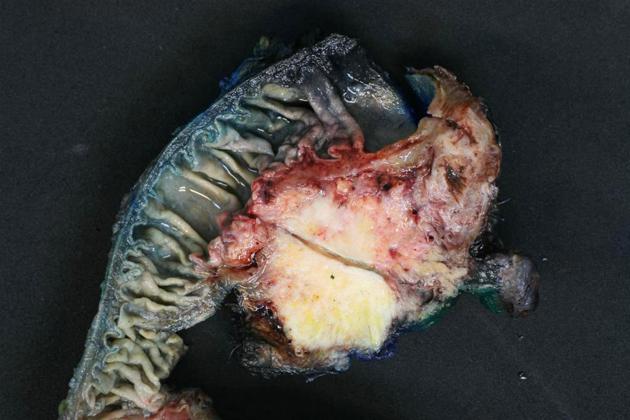
Cancerous cells arise from the pancreatic ductal epithelium. The tumour's histologic spectrum ranges from well-formed glandular/ductal structures in well-differentiated carcinoma to abortive tubular structures in poorly differentiated carcinoma in the background of desmoplastic stroma. Most glands in well-differentiated carcinoma produce mucin.
Two characteristic appearances of pancreatic adenocarcinoma are:
highly invasive behaviour of tumoural cells leads to infiltrating perineural spaces and blood vessels 12
explicit desmoplastic reaction, which causes hard consistency of tumour 12
Because of this tumour's aggressiveness, tumoural cells often directly extend into the spleen, adrenals, stomach, and transverse colon 12.
Location
head and uncinate process: two-thirds of cases
body and tail: one-third of cases 1
Subtypes
Histological subtypes include:
adenocarcinoma: majority
undifferentiated carcinoma and undifferentiated with osteoclast-like giant cells 12
Markers
The serum levels of these antigens are frequently raised in people with pancreatic cancer and can be used to track a patient's response to treatment. However, these markers cannot be used for population screening due to a lack of sensitivity and specificity 12.
Genetics
The most prevalent molecular changes responsible for pancreatic carcinoma are:
KRAS: the most commonly mutated oncogene (>90%)
TP53: alterations in 70-75%
SMAD4: inactivated in 55%
CKN2A: altered in 30% 12
Staging
Please see pancreatic ductal adenocarcinoma staging. Recurrence is probably better estimated by a risk score than by staging 10.
Radiographic features
As the majority of tumours (90%) 1 are not resectable, diagnosis is usually achieved with imaging (typically CT) although laparoscopy is often required to confirm resectability 1,2. The key to accurate staging is the assessment of the superior mesenteric artery and coeliac axis, which if involved exclude the patient from any attempted resection 1,2.
Fluoroscopy
Barium meal / small bowel follow-through
If large enough, may demonstrate a reverse impression on the duodenum: Frostburg inverted 3 sign or a wide duodenal sweep.
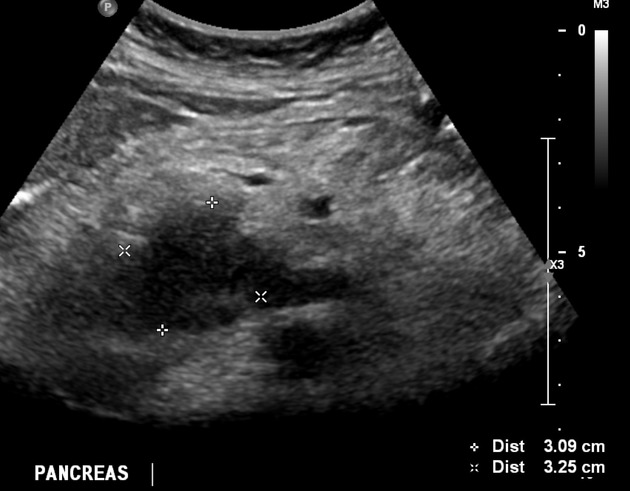
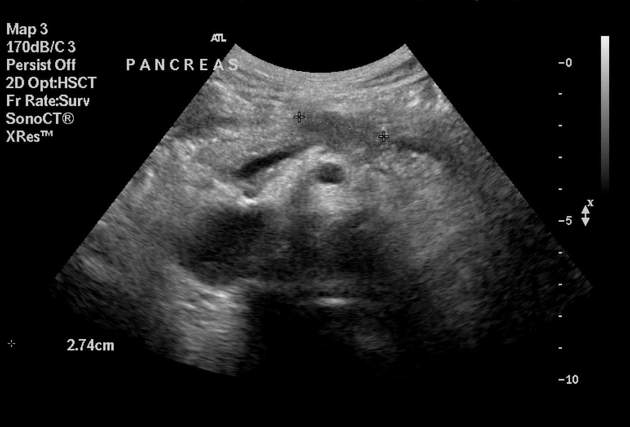
Ultrasound
Findings are non-specific and include:
hypoechoic mass
double duct sign may be seen
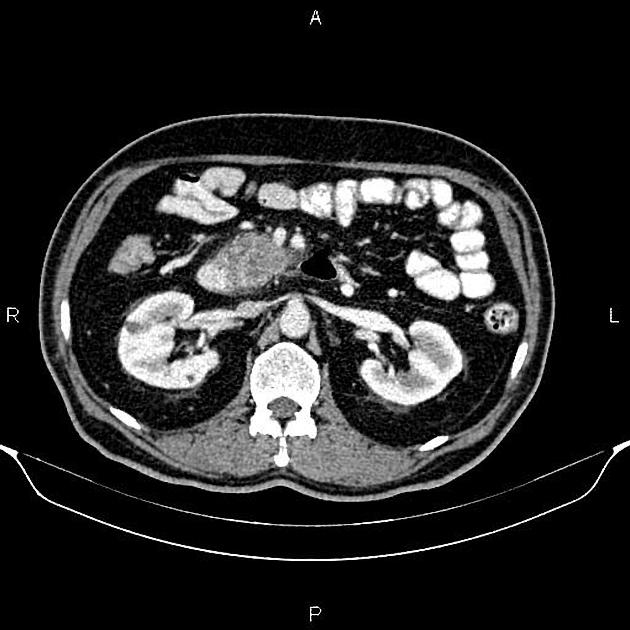
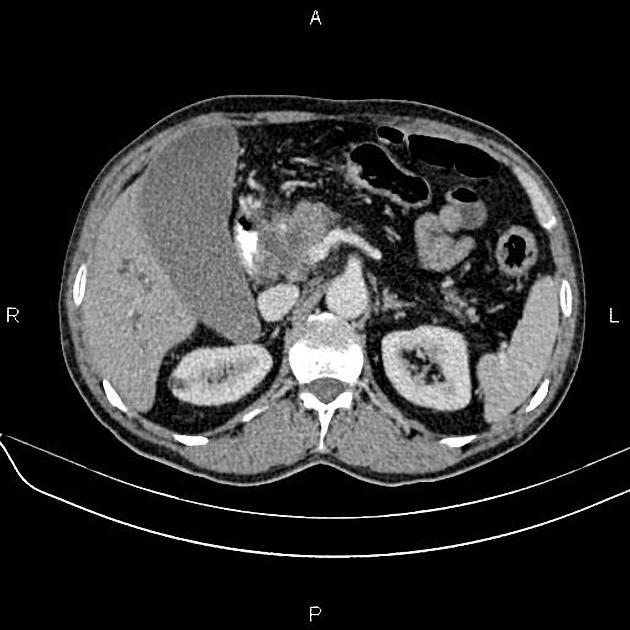
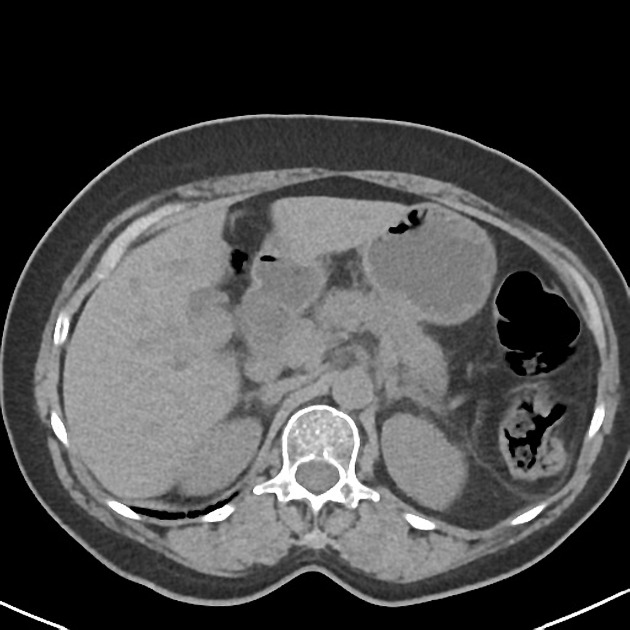
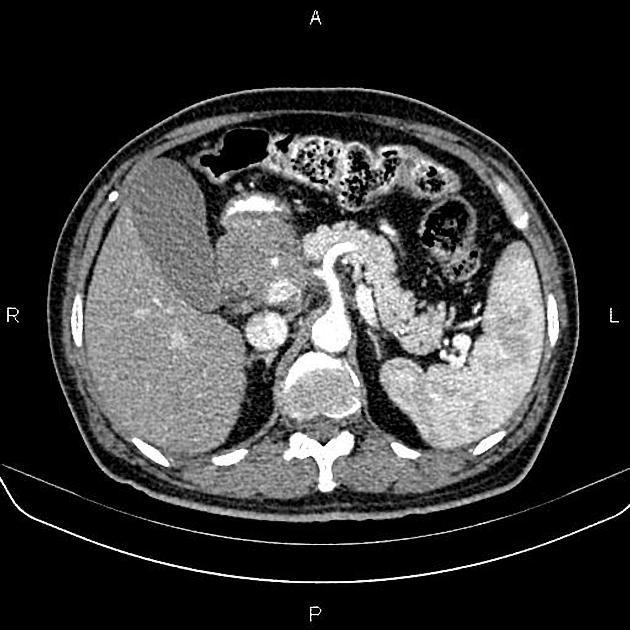
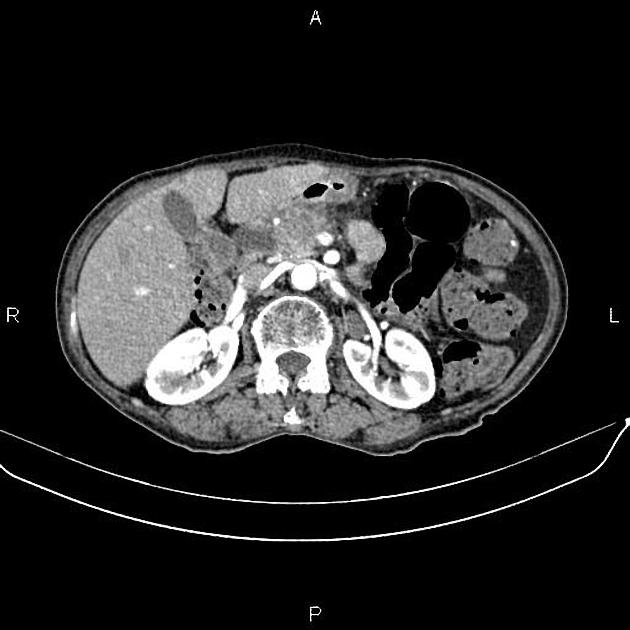
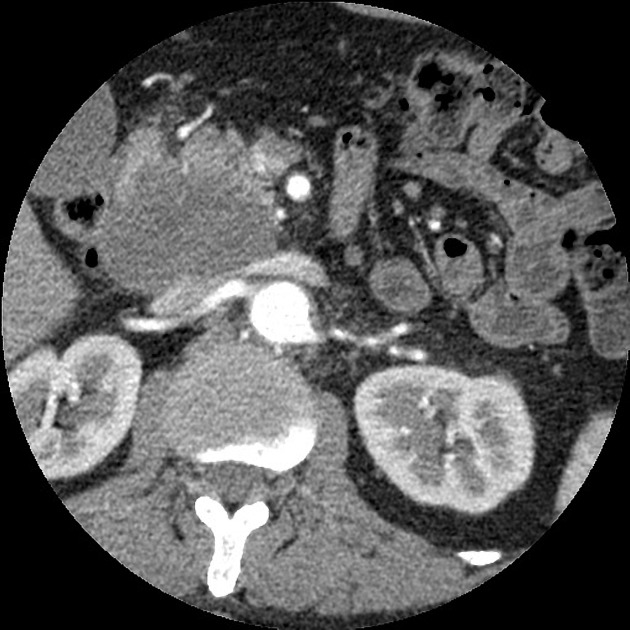
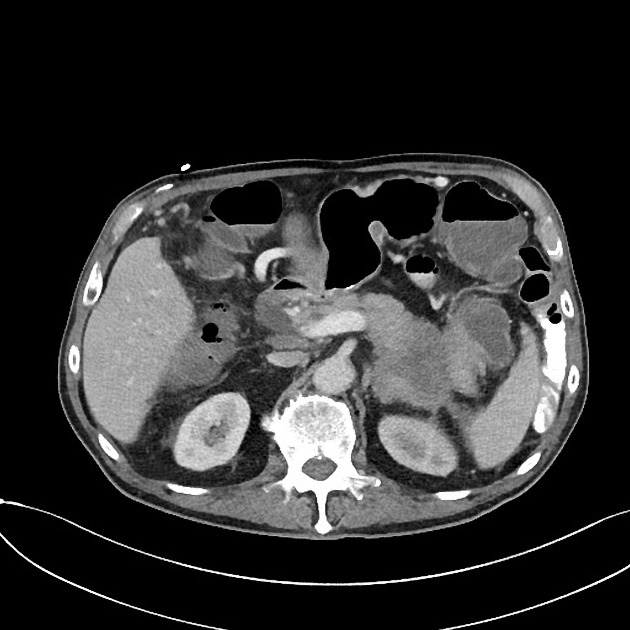
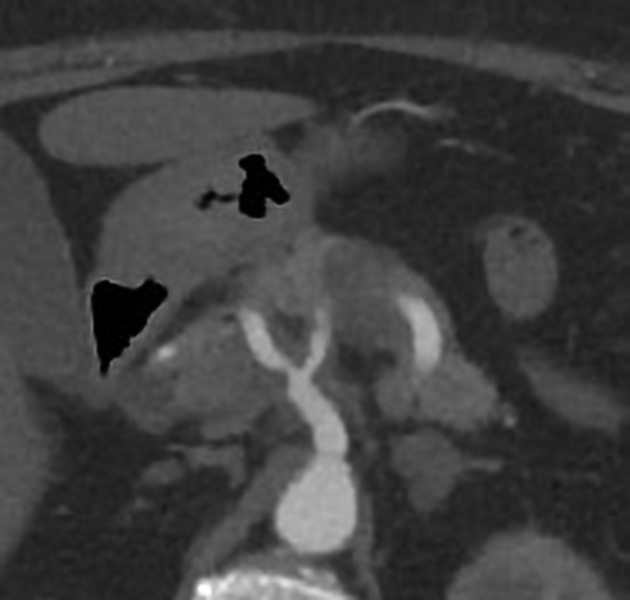
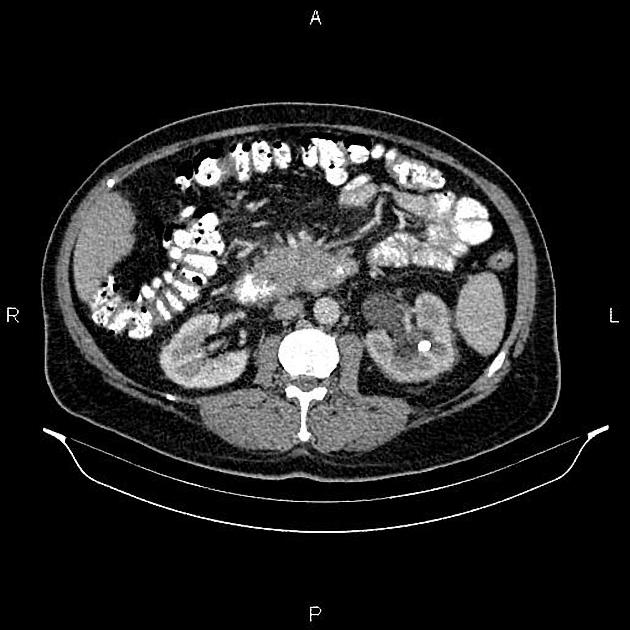
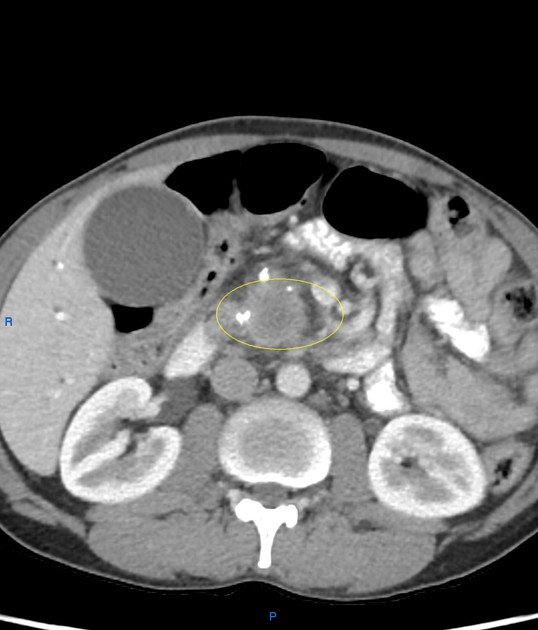
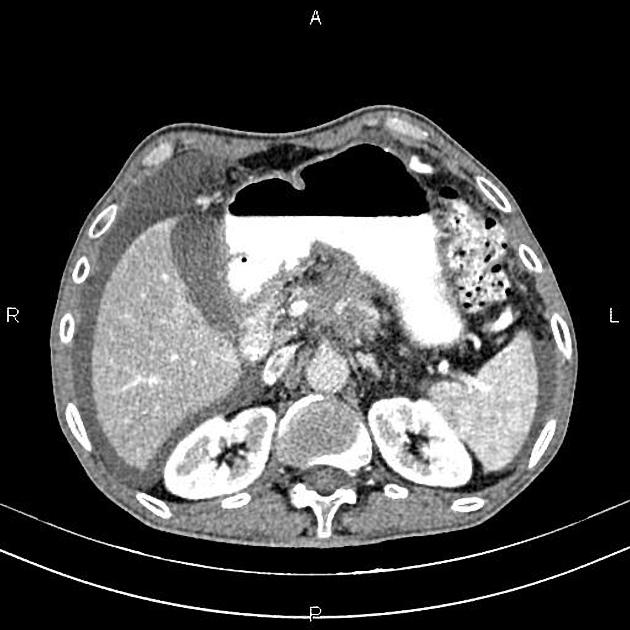
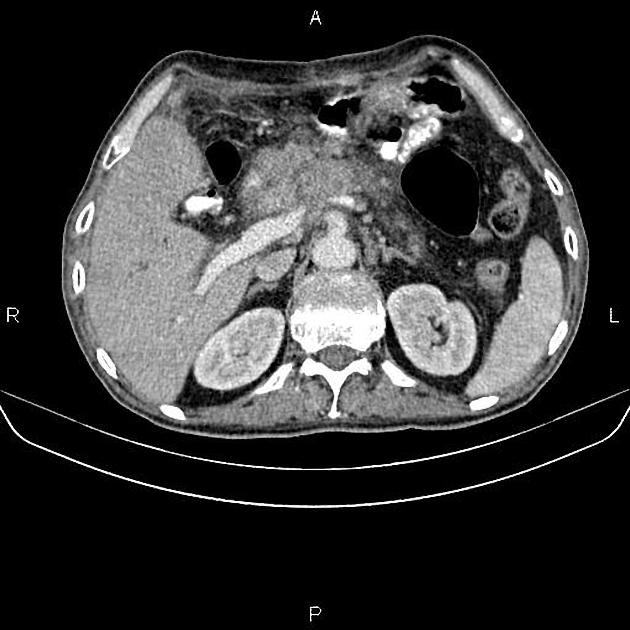
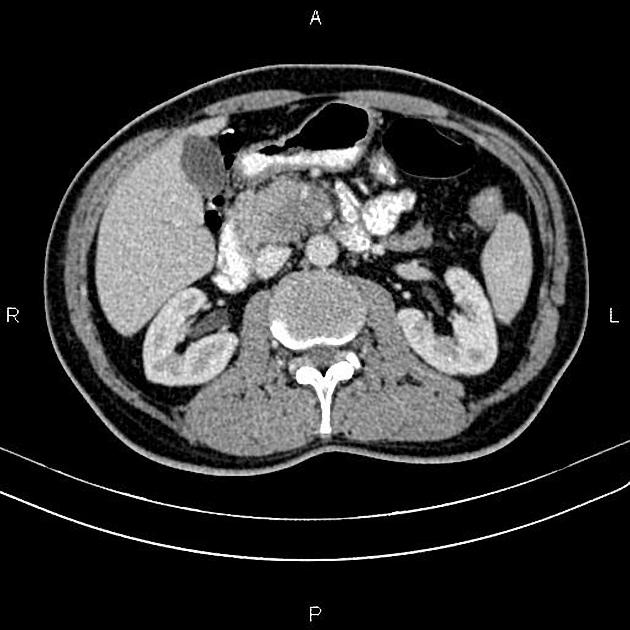
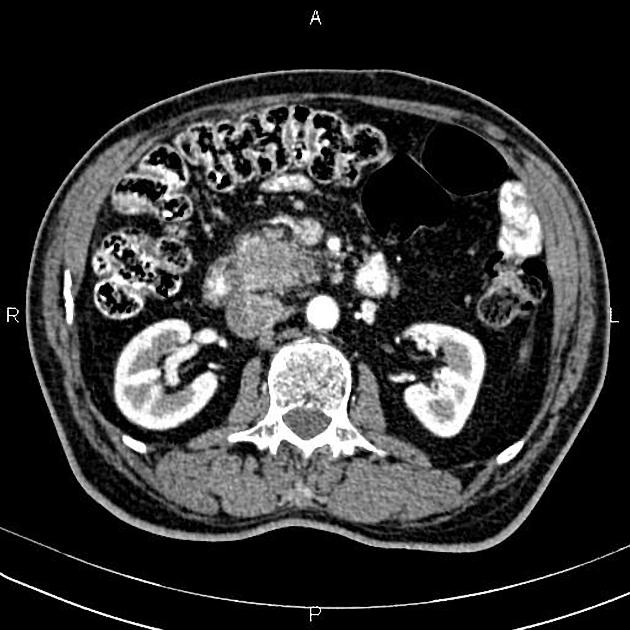
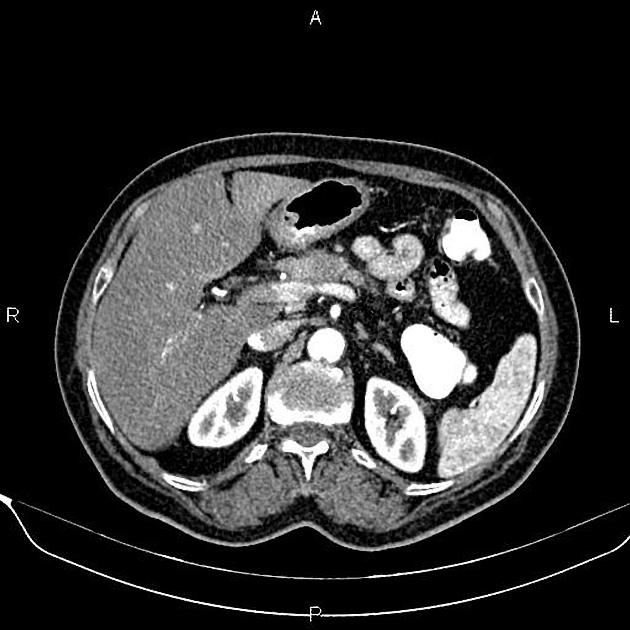
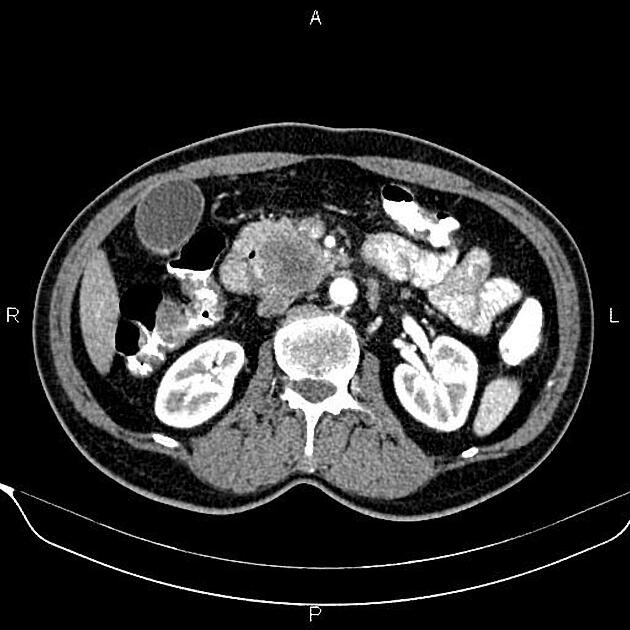
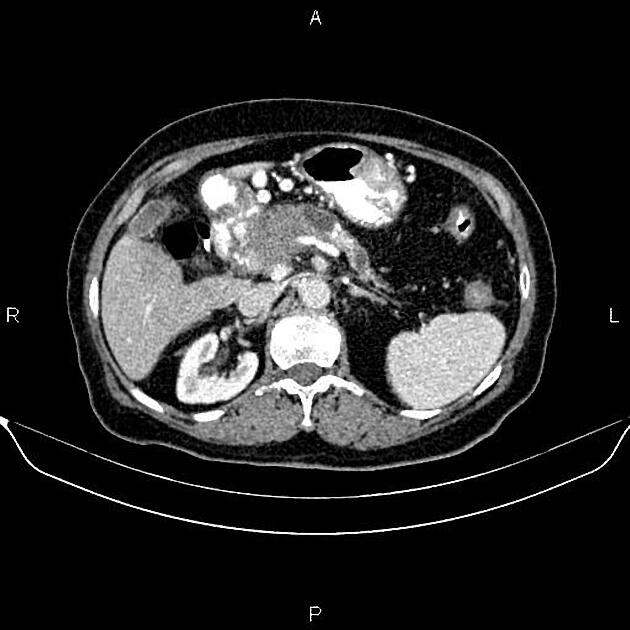
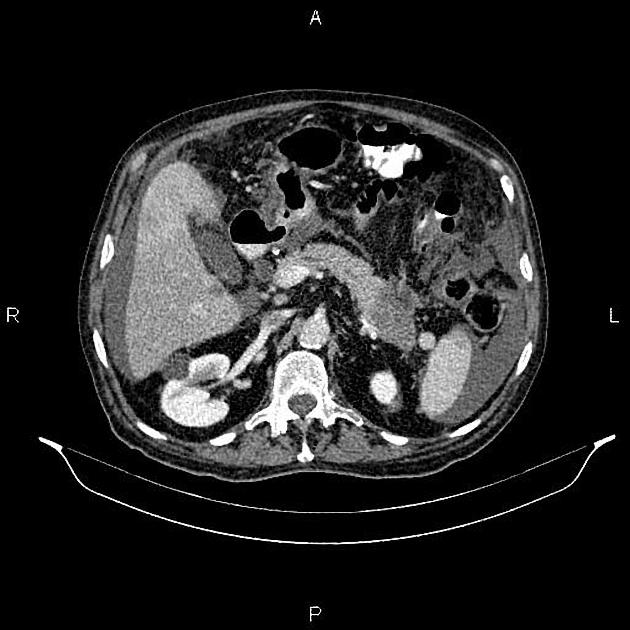
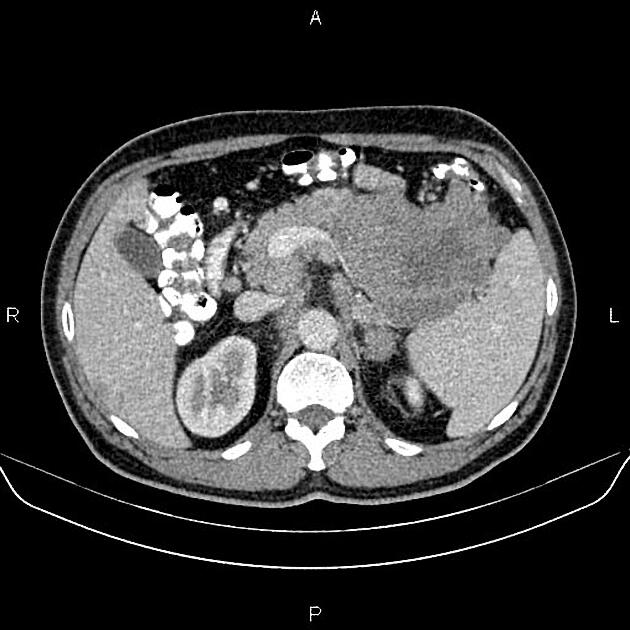
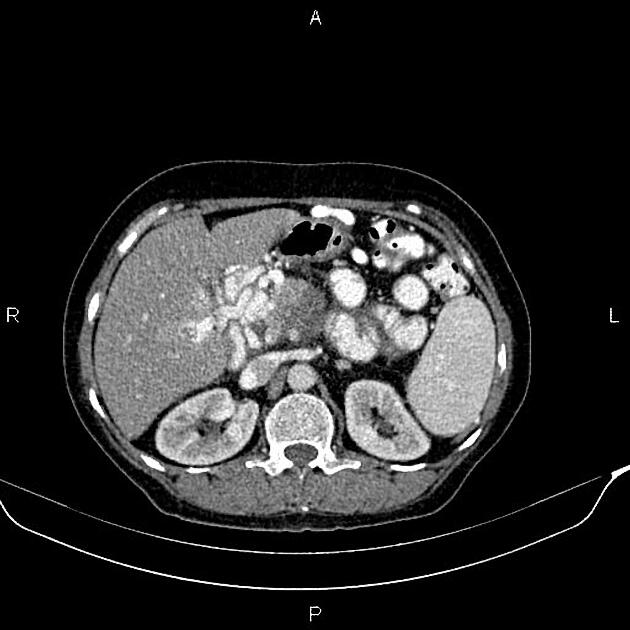
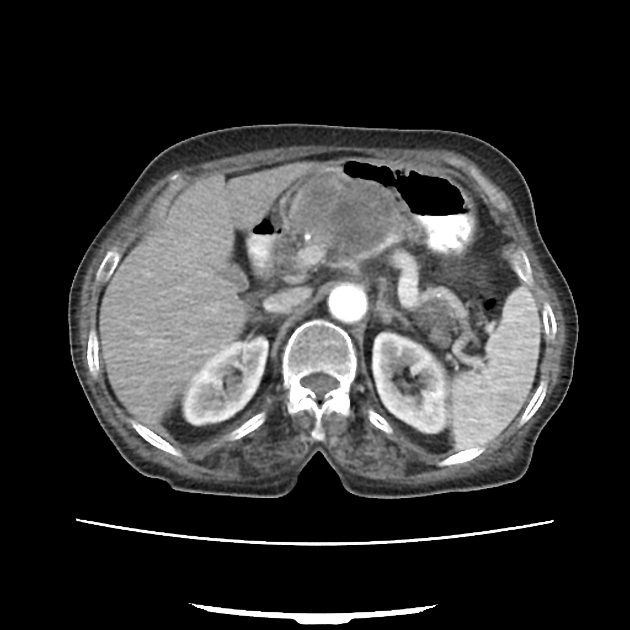
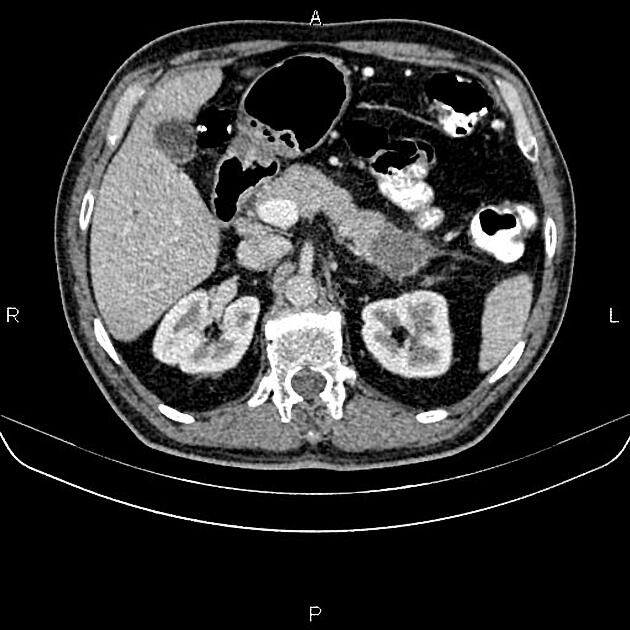
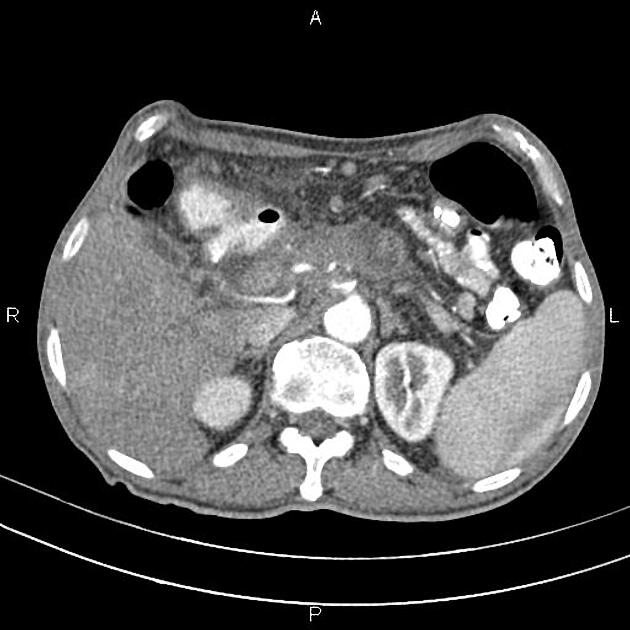
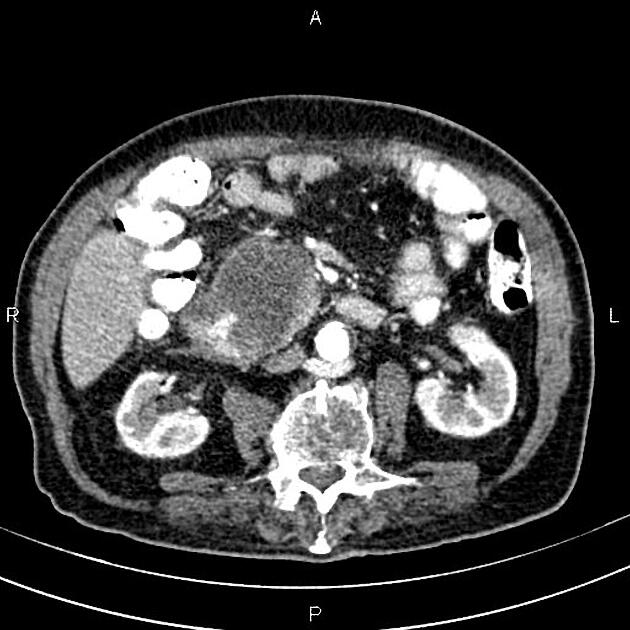
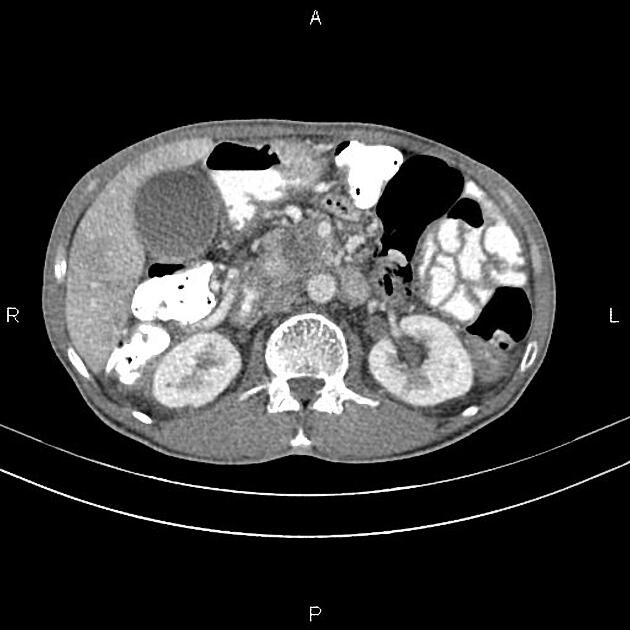
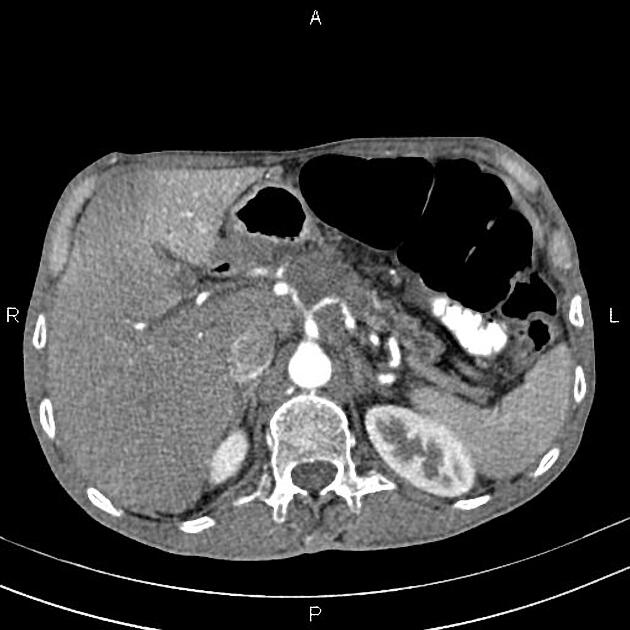
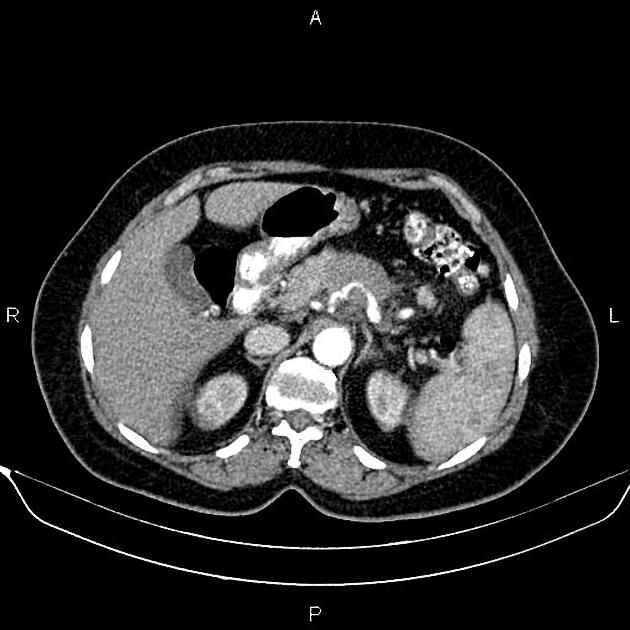
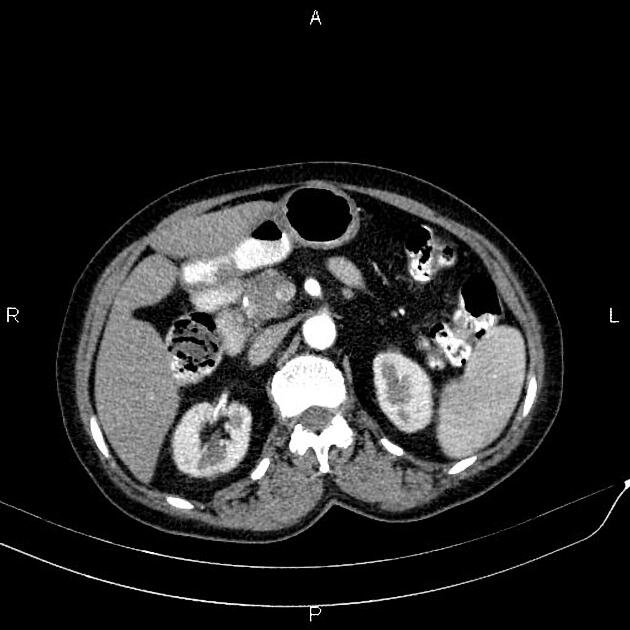
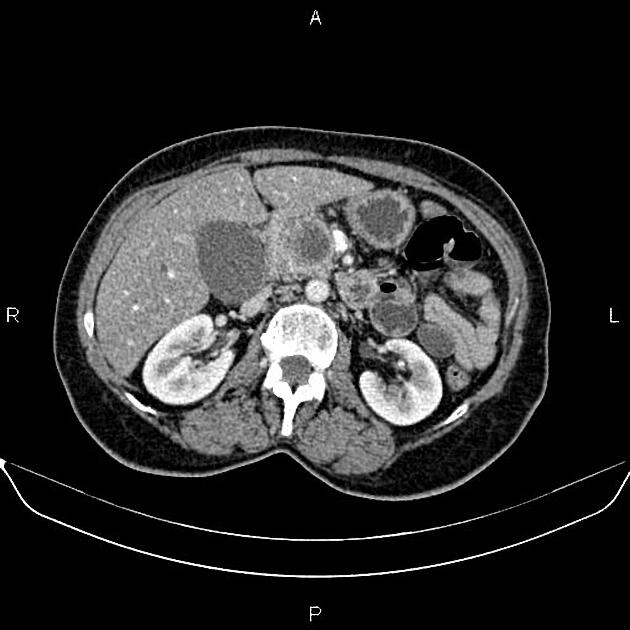
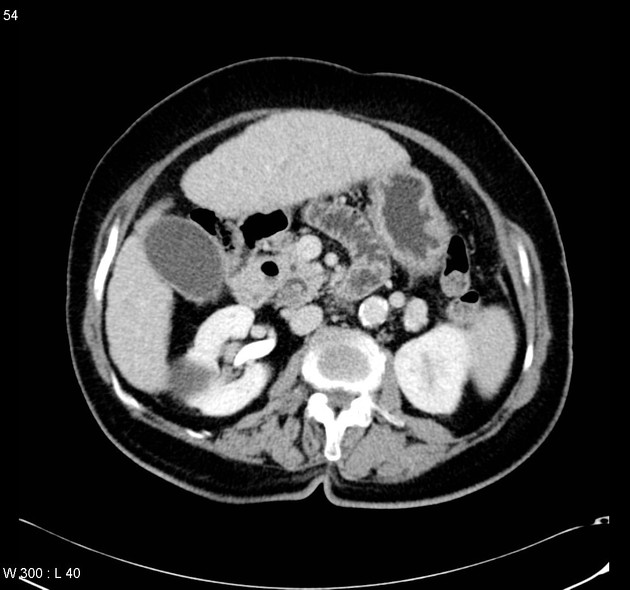
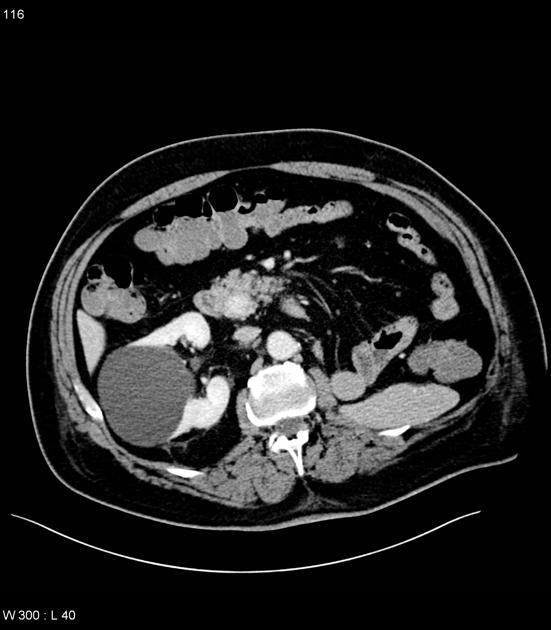
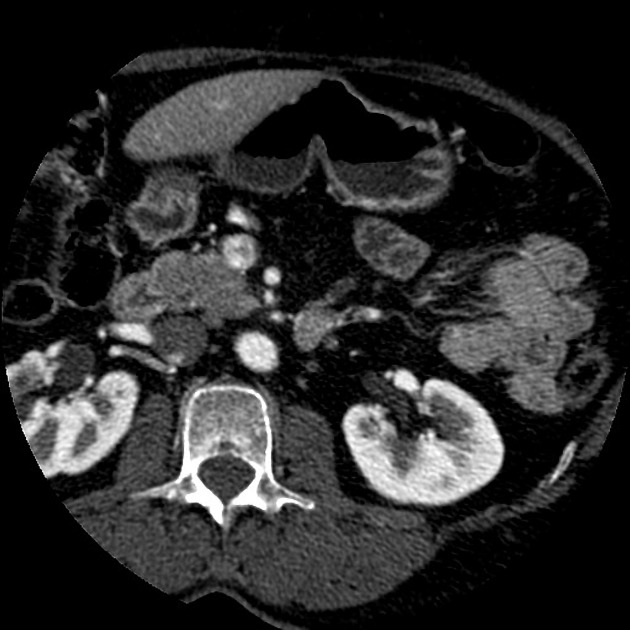
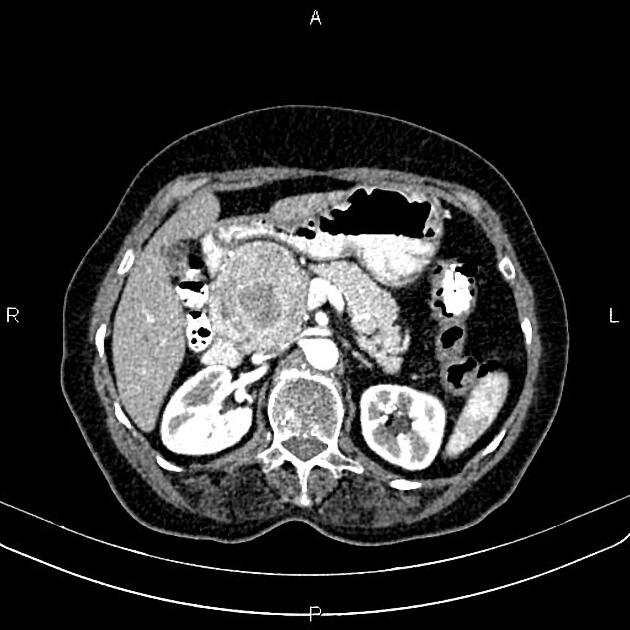
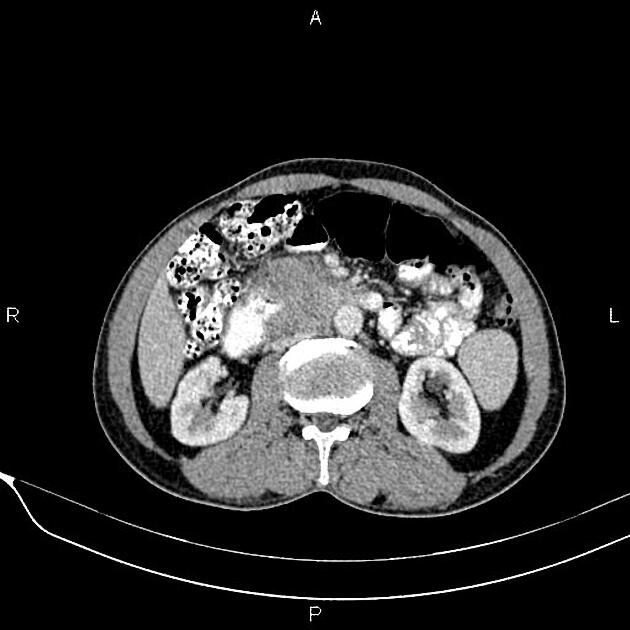
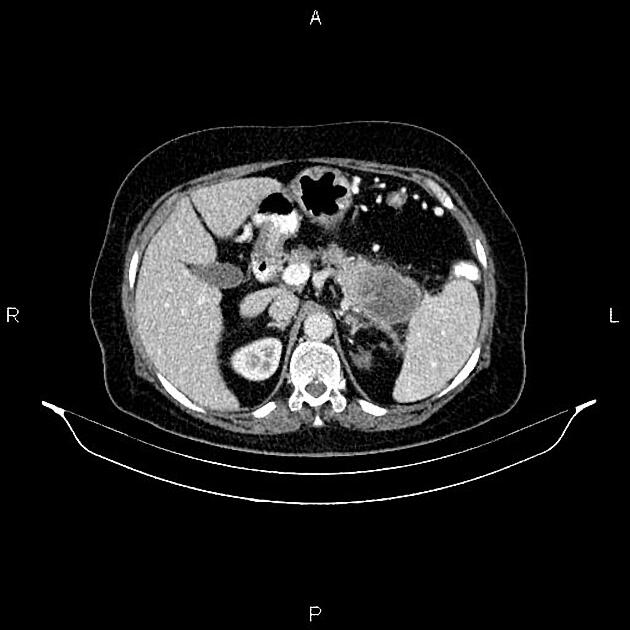
CT
-
role in imaging:
CT is the primary imaging modality ("workhorse") for pancreatic evaluation
-
ductal adenocarcinoma appearance:
poorly defined masses with extensive surrounding desmoplastic reaction
typically hypoattenuating on arterial phase scans in 75-90% of cases, with possible isoattenuation on delayed scans (necessitating multiphase imaging when pancreatic cancer is suspected)
double duct sign may be present
calcifications are rare and, when present, are more likely secondary to pre-existing conditions (e.g., chronic pancreatitis)
an enlarged pancreatic duct calibre to AP gland width ratio of ~0.5 may be present, reflecting ductal dilatation and parenchymal atrophy
-
assessment of resectability:
CT correlates well with surgical findings for predicting unresectability (positive predictive value: 89-100%)
-
the key feature is the tumour's relationship with surrounding vessels:
superior mesenteric artery and coeliac axis involvement: Tumour encasement of >180 degrees is classified as T4 disease, indicating unresectability
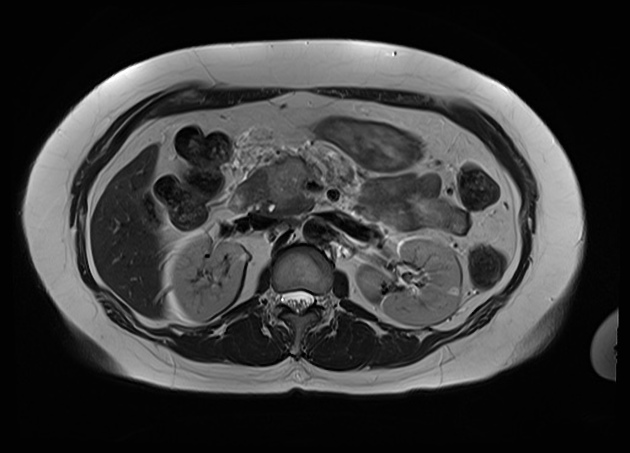
MRI
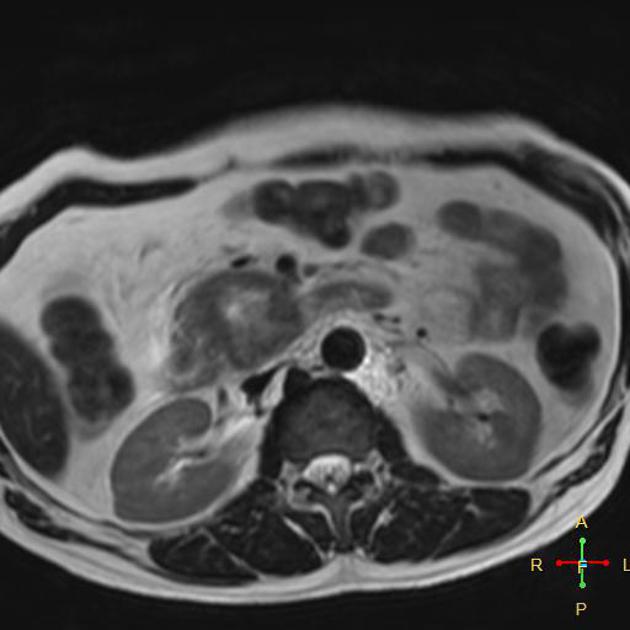
Signal characteristics include:
T1/T1FS: hypointense to normal pancreas 5
T1 C+ (Gd): slower enhancement than the normal pancreas, therefore dynamic injection with fat saturation with arterial phase imaging is ideal
T2/FLAIR: variable (therefore not very useful), depending on the amount of reactive desmoplastic reaction 1,5
MRCP: double duct sign may be seen
Treatment and prognosis
-
resectability:
the majority of pancreatic ductal adenocarcinomas are not resectable at the time of diagnosis
unresectability is primarily determined by the presence of metastasis and/or vascular invasion, particularly encasement of the coeliac trunk and superior mesenteric artery
-
surgical intervention:
surgical resection is potentially curative in stage I and II disease (see staging of pancreatic cancer); however, it is associated with significant morbidity (20-30%) and mortality (5%)3
resection of pancreatic head tumours is typically performed using the Whipple procedure
-
prognosis:
despite surgical resection, recurrence remains common, with only a modest improvement in overall survival (from 5% to 10% at 5 years)4
the approximate 12 month survival rate of pancreatic ductal adenocarcinoma is 25% from time of diagnosis
Differential diagnosis
General imaging differential considerations include:
-
the "duct penetrating sign" is an important sign to differentiate between chronic focal pancreatitis and pancreatic malignancy
pancreatic duct : parenchyma ratio is usually < 0.5
other pancreatic neoplasms
-
fatty infiltration of the pancreatic head
usually involving the anterior portion
no secondary signs (e.g. pancreatic duct or common bile duct dilatation)
high signal on T1 and signal drop on chemical shift sequences







 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.