Paroxysmal nocturnal hemoglobinuria (PNH), also known as Marchiafava-Micheli syndrome or Strübing-Marchiafava anemia, is an acquired hematopoietic stem cell disorder whereby some of the red blood cells produced are defective and are susceptible to premature destruction by the immune system, leading to complement-mediated hemolysis and hemoglobinuria.
On this page:
Terminology
The term comes from a mistaken 19th century belief that the hemolysis and subsequent hemoglobinuria occurred only intermittently (paroxysmally) and with greater frequency during the night (nocturnal). Hemoglobinuria is most prominent in the morning after the urine has concentrated overnight during sleep but hemolysis in paroxysmal nocturnal hemoglobinuria is actually a constant process.
Epidemiology
Prevalence is low, at 1-10 per 1,000,000. No difference in prevalence between the sexes has been found. Median age at diagnosis is in the fourth decade 2.
Clinical presentation
-
hemolytic anemia
fatigue
exertional dyspnea
hemoglobinuria, classically noticed in the morning
-
smooth muscle dystonia
dysphagia due to esophageal spasm
abdominal pain
Complications
Common
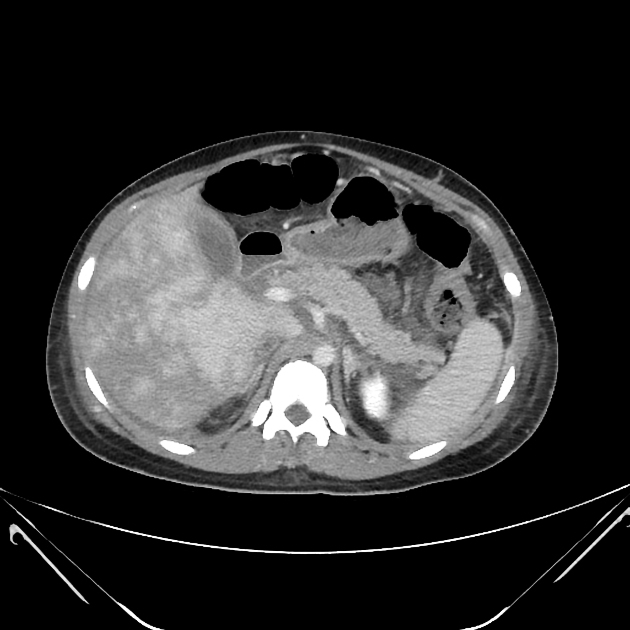
thrombosis, venous thrombosis being more common
renal impairment
Rare
bone marrow failure
myelodysplasia
acute leukemia
Pathology
Paroxysmal nocturnal hemoglobinuria is caused by a defect in surface proteins glycosylphosphatidylinositols (GPI) of red blood cells, typically due to an acquired mutation in the PIGA gene on the X chromosome in a hematopoietic stem cell 1,7. These surface proteins usually protect red blood cells and other immune cells from destruction via the complement system, thus a defect in these proteins increases their destruction, leading to the aforementioned clinical presentation 1.
In addition to the anemia from hemolysis, patients suffer from the direct effects of intravascular hemolysis that results in the absorption of nitric oxide, a key molecule in homeostasis, leading to smooth muscle dysfunction and platelet activation, markedly raising the risk of thrombosis 1.
Radiographic features
Radiographic features vary depending on the clinical presentation.
For example:
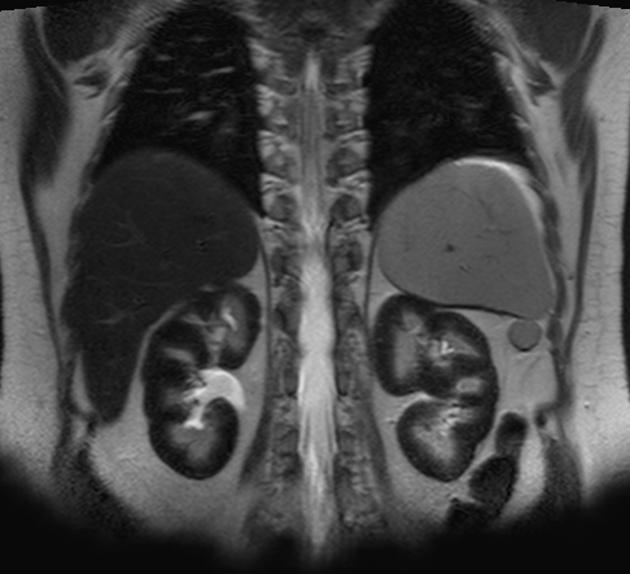
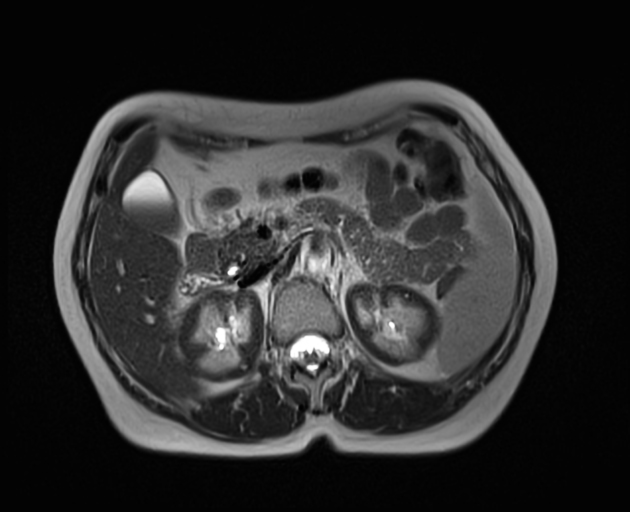
ultrasound, CTA and MRI/MRA may show features of thrombosis of major vessels, particularly in the abdomen
rarely thrombosis of smaller vessels may cause osteonecrosis of the femoral head
MRI of the abdomen may show renal hemosiderosis
Treatment and prognosis
Management options include:
-
supportive and/or symptomatic care:
blood transfusions
anticoagulation if thrombosis
-
disease-modifying treatments 1,2,5,6,9:
-
allogeneic haematopoetic stem cell transplant
only curative treatment available
C5 inhibitors: e.g. eculizumab, ravulizumab
C3 inhibitors: e.g. pegcetacoplan
-
Without therapy approximately 50% of patients die as a direct result of the disease. Many others are transfusion dependent for decades 3. Pregnancy results in extremely high risk for maternal and fetal mortality, predominantly resulting from thrombotic complications 4.
History and etymology
The condition was first described by German physician Paul Strübing in 1882, with further descriptions made by Italian physicians Ettore Marchiafava (1847-1935) and Ferdinando Micheli (1872-1936) 8.




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.