Presentation
50 year old female presenting with moderate intensity, non-radiating left upper thoracic back pain with exquisite paravertebral tenderness to palpation on exam. S/O "sweats" at onset. BP 185/82, VS otherwise wnl. Extremities warm, skin moist. Carotid and radial pulses 2+ and equal.
Patient Data
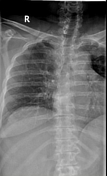
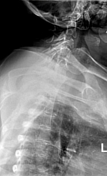
Chest radiograph without evidence of an acute cardiopulmonary process. Lungs fields clear bilaterally. Unremarkable cardiomediastinal silhouette.
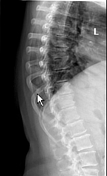
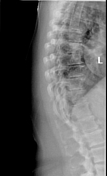
Thoracic spine radiographs demonstrate moderate diminution of intervertebral disc space height most prominently in the lower thoracic spine, accompanied by end plate spurring, consistent with mild-moderate degenerative changes.
Vitals unchanged at 1 hour reassessment, partial symptomatic improvement after administration of topical lidocaine (patch to affected area), oral diazepam, and acetaminophen. Appears in mild painful distress while quietly seated. Repeat physical exam unchanged from prior. Perform a focused bedside ultrasound of the heart, abdomen, and retroperitoneum.
Parasternal long axis view of the heart demonstrates a left ventricle with a normal chamber size and wall thickness with normal global systolic function. The right ventricular outflow tract, aortic root, and left atrium do not appear grossly dilated. No obvious endoluminal flap in the aortic root. Aortic and mitral valves demonstrate grossly normal excursion, coaptation, and structure.
Coronal and axial views of the right upper quadrant demonstrate no obvious hydronephrosis or intraperitoneal free fluid.
A sagittal view of the abdominal aorta demonstrates an undulating, hyperechoic linear band within the anechoic vessel lumen. Appears to move independently of the adjacent vessel wall. Visualized extent appears to have a normal diameter. Abovementioned findings highly suspicious for the intimal flap of an acute aortic dissection.
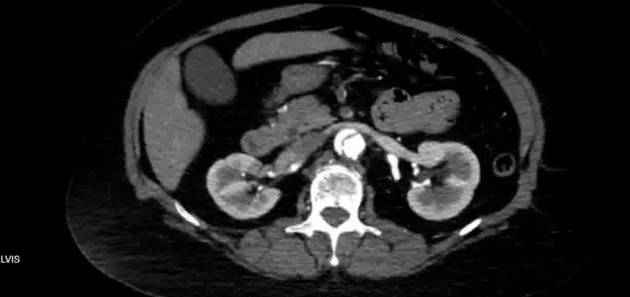
Demonstrates a Stanford B aortic dissection, with an intraluminal flap arising just distal to the left subclavian origin, extending through the descending and abdominal aorta to at least the bilateral common iliac arteries and likely the proximal external iliac arteries. Diameter of the ascending aorta is normal. No pericardial effusion.
The false lumen has a transverse diameter of 1.7 cm and has a segment of thrombus in the proximal 2 cm, but is otherwise patent. The true lumen is located medially, and measures 8 mm in transverse diameter. The intimal flap appears to involve the orifice of the superior mesenteric artery, with a segment of intraluminal thrombus involving a short length of the proximal SMA, flow reconstitution appears to occur distally.
The origins of the left innominate artery, left common carotid artery, left subclavian artery, celiac trunk, common hepatic artery, splenic artery, and inferior mesenteric arteries are patent. No intraperitoneal or pelvic free fluid, lymphadenopathy, or masses.
After diagnostic confirmation additional large bore vascular access was started, STAT surgical consult requested, and an esmolol bolus/infusion administered, titrating frequently to target a HR<60 and an SBP 100-120. Analgesia achieved with fentanyl pushes. A right radial arterial line was placed. Nicardipine drip started after failing to reach target with esmolol. Hemodynamic targets achieved shortly after nitroprusside infusion started. Transferred to surgical ICU for medical management.
Case Discussion
An acute aortic dissection classically presents with severe chest, abdominal, or back pain, of maximum intensity at or shortly after its appearance, and qualitatively described as "ripping" or "tearing." Concomitant appearance of pathology affecting disparate systems is also frequently described, such as focal neurologic deficits or acute limb ischemia 4.
Common risk factors include hypertension, bicuspid aortic valves, connective tissue diseases and vascular inflammation. It is defined by a disruption in the vascular intima which results in the creation of two vascular conduits (true and false lumens) separated by an intimal flap. The Stanford classification is often used to clinically subdivide patients based on the intimal flap's entry point proximal (type A) or distal (type B) to the left subclavian origin.
Bedside transthoracic echocardiography is often the first imaging modality employed in the emergency department, and while incompletely sensitive can quickly identify complications requiring intervention and/or emergent consultation. While transesophageal echocardiography has remarkable test characteristics for the diagnosis of aortic dissection, the urgency of the clinical situation often precludes an invasive procedure requiring sedation 4.
Sonographic features of an acute aortic dissection include:
- subdivision of the vessel by a hyperechoic, mobile, linear intraluminal structure (intimal flap)
- the true lumen tends to be smaller, increasing in diameter during systole 1
- the larger false lumen may demonstrate intraluminal thrombus or spontaneous echocontrast
- color flow Doppler interrogation may reveal alternation of filling corresponding to the cardiac cycle, as well as aliasing at intraluminal points of communication
- aortic root dilation
- may result in annular dilation and aortic valve regurgitation
Complications should be specifically sought, including:
-
aortic valve regurgitation
- may also occur due to leaflet prolapse
- myocardial ischemia
- secondary to coronary involvement, most often the right coronary artery
- failure of (or delayed) systolic thickening of the myocardium pertaining to the affected distribution is suggestive
- pericardial effusion with or without tamponade
- often with some degree of mixed echogenicity
The case patient had well filled cardiac chambers without an effusion, normal valvular coaptation and aortic root diameter, and visualized left ventricular segments (basal to mid anteroseptum and inferolateral walls) were normokinetic, lowering the suspicion for incipient complications to arise.
Contrast enhanced CT should be sought expeditiously as the modality of choice to to evaluate for an acute aortic dissection. As with this case it allows identification of the true and false lumens, noting the following salient features:
- the beak sign, quite distinct just caudad to the diaphragmatic hiatus in the above CT, identifies the sharp angulation at the periphery of the false lumen
- lower intraluminal density with patchy thrombosis and delayed opacification are notable in the false lumen in the above case
- the left renal artery is also seen to characteristically arise from the false lumen




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.