Diffuse midline glioma, H3 K27-altered is a specific entity that represents the majority of diffuse intrinsic pontine gliomas, although identical tumors are also found elsewhere in the midline (e.g. brainstem, spinal cord and thalamus) 1. They are aggressive tumors with a poor prognosis and are considered WHO grade 4 tumors regardless of histological features 1,3,4.
On this page:
Epidemiology
The majority of these tumors are found in young children and are located in the pons 3. They are also seen elsewhere in the midline. In such cases, they tend to be encountered in children and young adults. This is particularly the case with spinal cord tumors that are seen primarily in young adults 3.
Clinical presentation
The clinical presentation depends upon the location of the tumor. Typically patients with brainstem tumors present with multiple cranial nerve palsies, depending on the location of the tumor, and signs of raised intracranial pressure. Cerebellar signs may also be elicited including ataxia, dysarthria, nystagmus and sleep apnea.
Pathology
Diffuse midline glioma, H3 K27-altered was first added to the 2016 update of the WHO classification of CNS tumors and is now considered one of the pediatric-type diffuse high-grade gliomas 3,4.
Location
Diffuse midline glioma, H3 K27-altered can be found throughout the midline structures of the central nervous system:
thalamus
-
brainstem
mesencephalic
pontine: most common
medullary
spinal cord
Microscopic appearance
Diffuse midline gliomas, H3 K27-altered usually appear histologically as astrocytic tumors. In a minority of cases they appear histologically low-grade (without mitotic figures, microvascular proliferation or necrosis), however, even then they are considered WHO grade 4 tumors 3,4.
Immunophenotype
S100: positive
NCAM1: positive
OLIG2: positive
H3F3A K27M mutation: usually positive, although other mutations are also recognized (see below)
p53 protein: positive in 50%
GFAP: variable
chromogranin-A: negative
NeuN: negative
Importantly, unlike adult diffuse gliomas, mutations of IDH, CDKN2A and/or CDKN2B deletions, TERT promoter mutations, and MGMT promoter methylation are rare in diffuse midline gliomas, H3 K27-altered 4.
Genetics
Genomic work has uncovered distinct mutations found in the majority of diffuse midline gliomas resulting in the inclusion of diffuse midline glioma H3 K27-altered as a distinct entity and the removal of diffuse intrinsic pontine gliomas from the WHO classification of CNS tumors 1,3.
These mutations are in the histone H3F3A gene (K27M mutations) or less frequently HIST1H3B and HIST2H3C genes 2,3.
A number of other distinct but related histone gene mutations have also been identified in similar tumors 2.
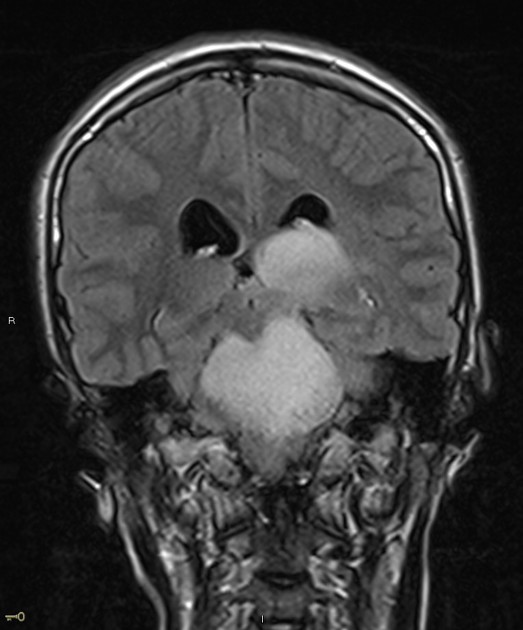
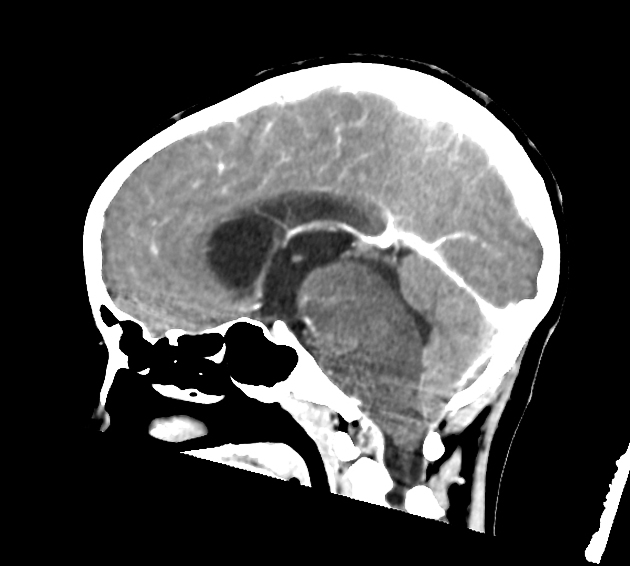
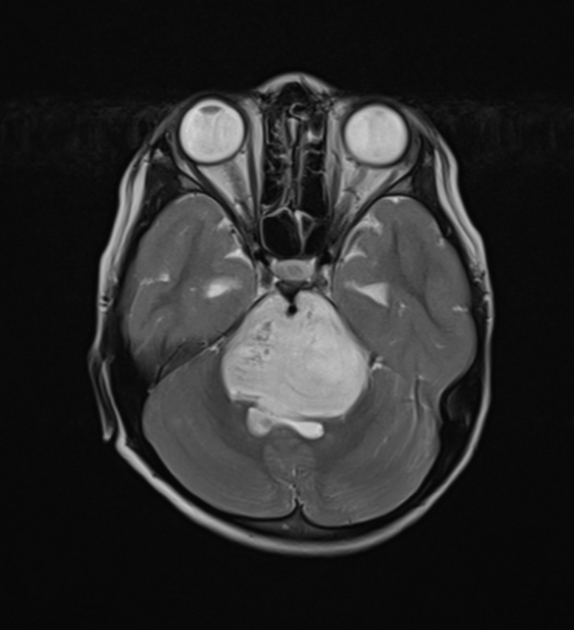
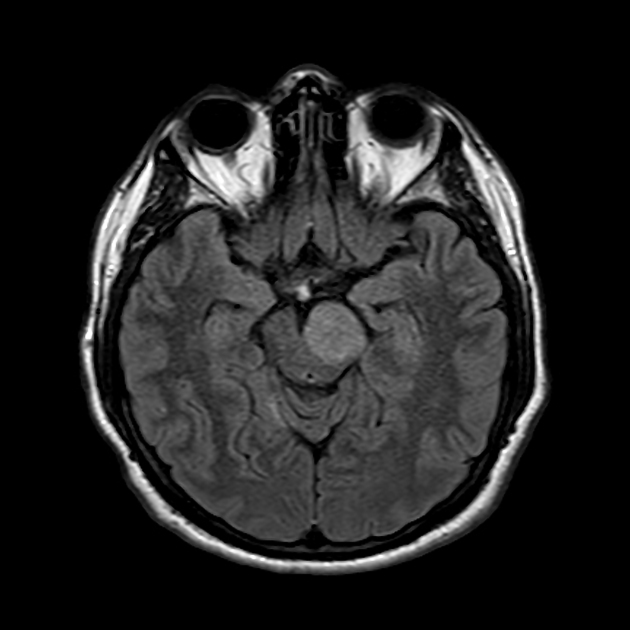
Radiographic features
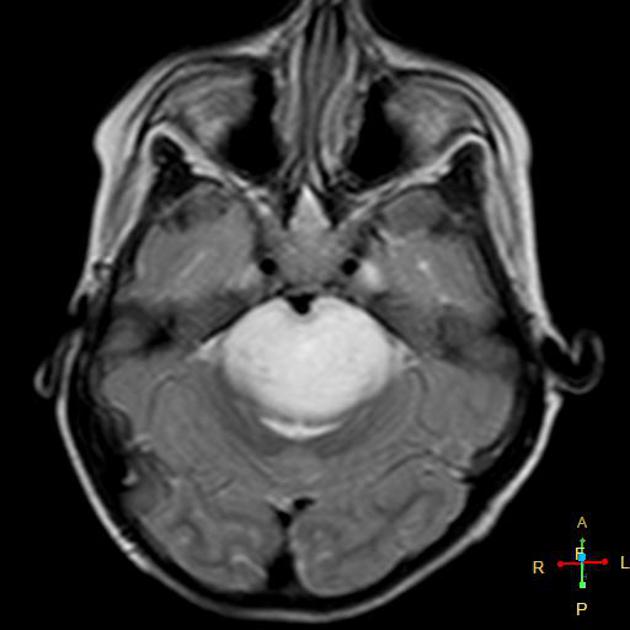
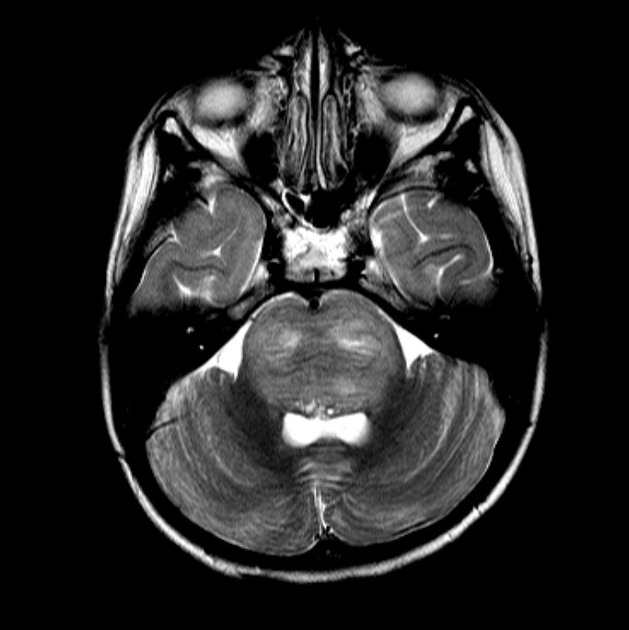
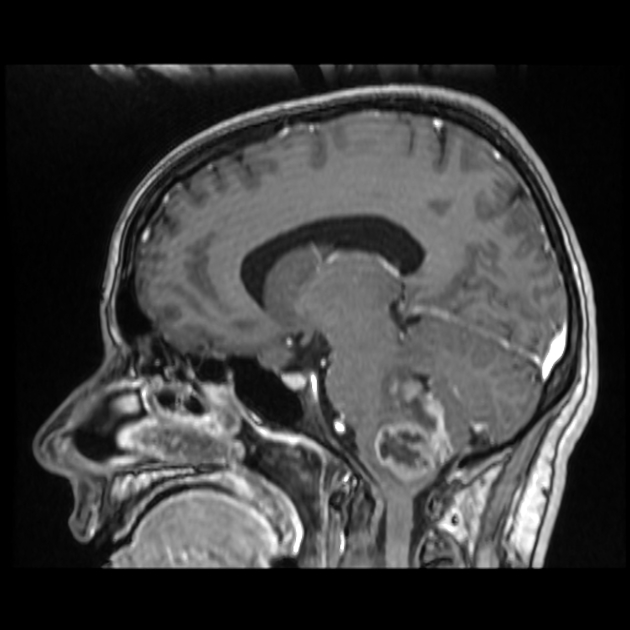
In tumors located within the pons, the pons is enlarged with the basilar artery displaced anteriorly against the clivus and potentially engulfed. The floor of the fourth ventricle is flattened ("flat floor of fourth ventricle sign") and obstructive hydrocephalus may be present. Occasionally the tumor is exophytic, either outwards into the basal cisterns or centrally in the 4th ventricle.
Usually, the tumor is homogeneous pre-treatment, however, in a minority of patients areas of necrosis may be present.
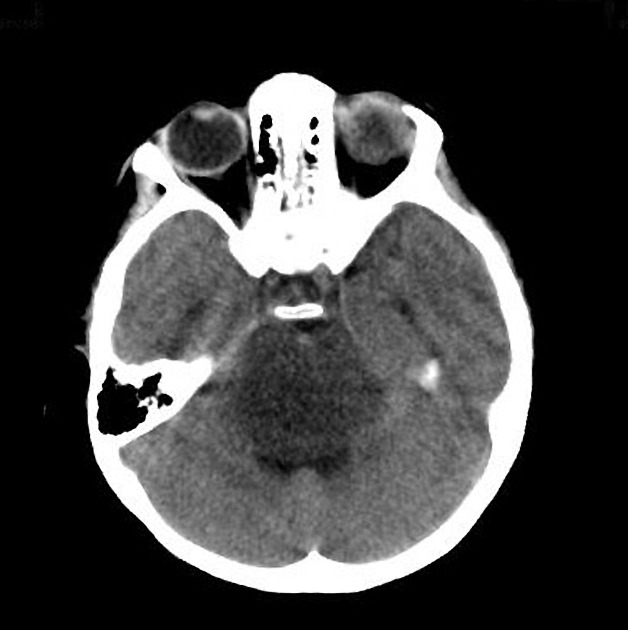
CT
Typically hypodense with little, if any, enhancement.
MRI
T1: hypointense
-
T2:
hyperintense mass
relative hypointense areas may indicate foci of higher cellularity or spared white matter tract 5
-
T1 C+:
usually non-enhancing
focal enhancing areas (correlated with relative hypointensity on T2 and low ADC values) may associate with higher grade areas 5
enhancement can be seen post-radiotherapy
-
DWI/ADC:
typically facilitated diffusion
some areas of diffusion restriction may represent areas of higher grade 5
T2*/SWI: areas of marked hypointensity/blooming in cases of intralesional hemorrhage 5
DTI: disruption of the white matter tracts indicates diffuse midline glioma rather than focal brain stem gliomas 5
The presence of restricted diffusion at non-enhancing DMG is called DWI-Gd mismatch sign 6, and it is found to be a very poor prognostic sign.
Extensive spread is relatively frequent, both craniocaudally to involve the cerebral hemispheres and spinal cord, as well as leptomeningeal spread 3.
Treatment and prognosis
Due to the high rate of severe complications with biopsy, treatment has historically been commenced without histological confirmation, although due to the identification of distinct mutations (K27M mutations in the histone H3 gene H3F3A and related HIST1H3B genes), stereotactic biopsy is being performed in some centers, and may become routine when therapies specifically targeted to these mutations become available 2.
Prognosis remains poor, with a 2-year survival of less than 10% 3.





 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.