Huntington disease (HD), also known as Huntington chorea, is an autosomal dominant trinucleotide repeat neurodegenerative disease characterized by a loss of GABAergic neurons of the basal ganglia, especially atrophy of the caudate nucleus and putamen (dorsal striatum). Huntington disease is clinically characterized by progressive unintentional chorea, subcortical type cognitive impairment, behavioral changes, and depression which starts in midlife.
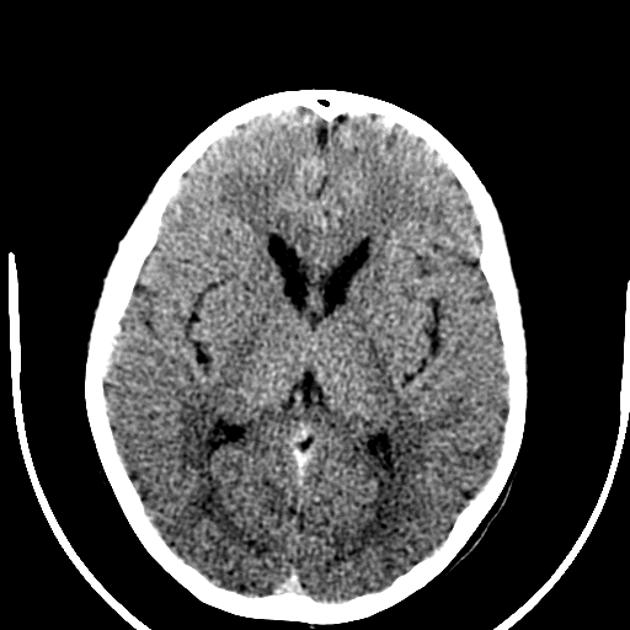
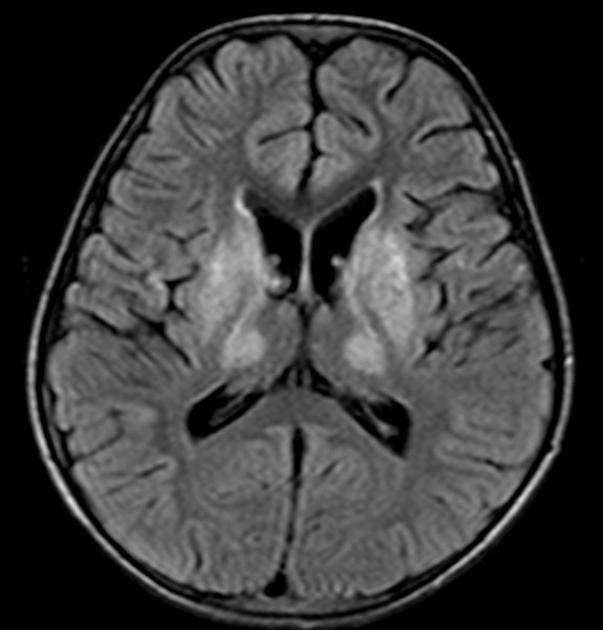
On imaging, it is classically characterized by atrophy of the caudate nucleus with concomitant enlargement of the frontal horns of the lateral ventricles.
On this page:
Epidemiology
Huntington disease typically has a prevalence of 2-10 per 100,000 and is typically diagnosed between 30 and 50 years of age 3. Incidence is equal in both genders, although there appears to be an effect depending on the gender of the parent from whom the defect was inherited: if inherited from the father, presentation is earlier. The cause for this effect is as yet uncertain 3.
In approximately 1-6% of patients, symptoms occur before the age of 20 years, the so-called 'juvenile' or Westphal form, which appears to be a variant of the usual adult form, with a different pattern of symptoms. In juvenile cases having inherited the disease from the father is far more common 3.
Clinical presentation
The clinical presentation may be broad, encompassing a range of motor and non-motor manifestations 13.
Motor manifestations 13:
chorea and motor impersistence ('negative chorea')
parkinsonism: bradykinesia, rigidity
imbalance and unsteadiness
dysphagia
other: dystonia, tics, myoclonus
Non-motor manifestations 13:
cognitive impairment
-
psychiatric manifestations
-
depression and anxiety
markedly increased (10-fold increase) suicide rate
agitation and irritability
psychosis
apathy
-
weight loss
In the the juvenile (or Westphal) variant, the clinical presentation has an emphasis on early parkinsonism, cognitive impairment, ataxia, and myoclonus 13.
Pathology
Genetics
The mutation responsible is on chromosome 4p16:3 and consists of a CAG trinucleotide repeat, which normally encodes for the huntingtin protein. The inheritance pattern of Huntington disease is autosomal dominant with complete penetrance and genetic anticipation (i.e. next generation will have more repeats of CAG and a more severe course of the disease or show symptoms earlier) particularly if the inherited mutated allele is paternal. The usual <27 repeats are amplified to >36, and the greater the number of repeats the earlier the age of onset, such that patients with the juvenile (or Westphal) variant often have >60 repeats 3,13.
Macroscopic appearance
Microscopically, there are Huntington nuclear inclusion bodies 8. These intranuclear inclusions are ubiquitin and Huntingtin exon 1-encoded protein fragments 12. Both deep grey matter and, to a lesser degree, white matter are involved in HD. The absence of striatal neurons, which function to reduce motor activity, lead to increased motor output, so cause to choreoathetosis 11.
Radiographic features
Although all modalities capable of structural brain imaging will demonstrate morphological changes of Huntington disease, MRI has the greatest spatial and contrast resolution and is thus preferred.
MRI
The most striking and best-known feature is that of caudate head atrophy. There is also, however, prominent putaminal volume loss which is usually not as easily recognized on visual inspection but seen well on morphometry 9. This is particularly the case in younger patients 4. The combination results in enlargement of the frontal horns, often giving them a "box" like configuration 2-4.
This can be quantified by a number of measurements:
frontal horn width to intercaudate distance ratio (FH/CC), which is reduced in Huntington disease
intercaudate distance to inner table width ratio (CC/IT), which is increased in Huntington disease
In some cases, the basal ganglia may show decreased T2 signal and blooming on SWI in keeping with iron deposition 7. Generalized age-inappropriate cortical thinning and volume loss is also recognized 4,14.
MR spectroscopy may demonstrate elevation of lactate, although this can be variably present in location (most often in the basal ganglia) 15,17. There is also a decrease in NAA/creatine ratio, particularly in the basal ganglia, in-keeping with neuronal loss 15,16.
Nuclear medicine
PET
PET demonstrates hypometabolism by decreased FDG uptake in basal ganglia and frontal cortex even before noticeable caudate nucleus volume loss 6.
Treatment and prognosis
No disease-modifying treatment is generally available 4,13. Symptomatic treatment is available for individual manifestations of the condition, for example, for chorea or depression 13.
The adult-onset form is slower in its course and inevitably leads to death in 14-15 years, whereas the juvenile form has a more rapidly progressive course, with death occurring in 7-8 years 3,13.
History and etymology
The condition is named after George Huntington (1850-1916), an American physician 1.
Differential diagnosis
The main differential for caudate atrophy is the following:
neuroacanthocytosis syndromes such as chorea-acanthocytosis or McLeod syndrome
frontotemporal lobar degeneration, specifically the fused in sarcoma protein variant (FTLD-FUS) 10
Other differential considerations:
Wilson disease: more commonly involves white matter, thalamus, cerebellum and brainstem 3
Leigh disease: more commonly involves white matter, thalamus, cerebellum and brainstem

















 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.