Melanoma cancer staging refers to TNM classification of primary cutaneous melanoma. The system does not apply to the primary non-cutaneous melanomas. The following article reflects the 8th edition manual published by the American Joint Committee on Cancer (AJCC), which has been used for staging since January 1, 2018 1.
Primary tumor (T)
TX: primary tumor thickness cannot be assessed
T0: no evidence of primary tumor
Tis: melanoma in situ, including lentigo maligna and superficial spreading melanoma
-
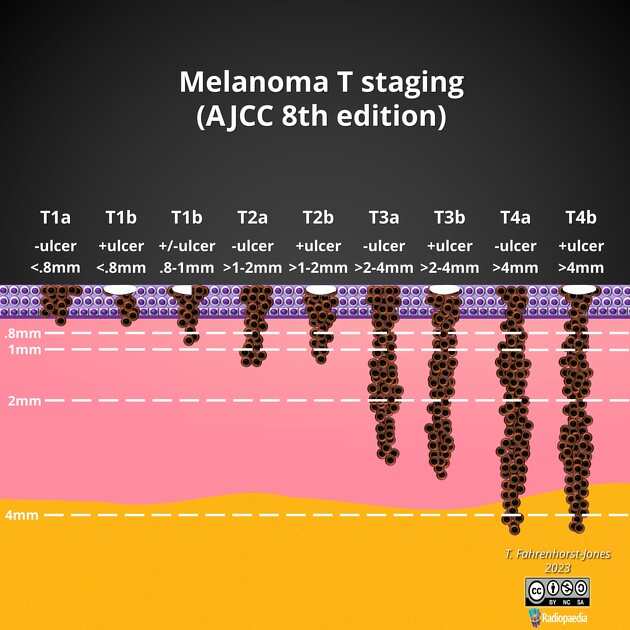
T1: ≤1 mm in maximum thickness recorded to nearest 0.1mm (not nearest 0.01 mm)
T1a: <0.8 mm without ulceration (U-)
T1b: <0.8 mm with ulceration (U+) or 0.8-1.0 mm with or without ulceration
-
T2: >1 mm but ≤2 mm
T2a: >1.0-2.0 mm without ulceration
T2b: >1.0-2.0 mm with ulceration
-
T3: >2.0 mm but ≤4.0 mm
T3a: >2.0-4.0 mm without ulceration
T3b: >2.0-4.0 mm with ulceration
-
T4: >4.0 mm
T4a: >4.0 mm without ulceration
T4b: >4.0 mm with ulceration
Regional lymph node (N)
N0: no evidence of nodal metastasis (by imaging, clinical examination or pathological evaluation)
-
N1: one involved node or any in-transit (ITM), satellite, and/or microsatellite metastases with no tumor-involved nodes
N1a: one clinically occult node (i.e. detected by sentinel lymph node biopsy SLNB). No ITM/satellite/microsatellites
N1b: one clinically detected node. No ITM/satellite/microsatellites
N1c: no regional node disease but presence of in-transit (ITM), satellite, and/or microsatellite metastases
-
N2: 2 or 3 tumor-involved nodes or in-transit, satellite, and/or microsatellite metastases with 1 tumor-involved node
N2a: 2 or 3 clinically occult (i.e. detected by SLNB). No ITM/satellite/microsatellites
N2b: 2 or 3, at least 1 of which was clinically detected. No ITM/satellite/microsatellites
N2c: 1 clinically occult or clinically detected with presence of in-transit (ITM), satellite, and/or microsatellite metastases
-
N3: ≥4 tumor-involved nodes or in-transit, satellite, and/or microsatellite metastases with ≥2 tumor-involved nodes, or any number of matted nodes without or with in-transit, satellite, and/or microsatellite metastases
N3a: ≥4 clinically occult (i.e. detected by SLNB). No ITM/satellite/microsatellites
N3b: ≥4, at least 1 of which was clinically detected, or presence of any number of matted nodes. No ITM/satellite/microsatellites
N3c: ≥2 clinically occult or clinically detected and/or presence of any number of matted nodes with presence of in-transit (ITM), satellite, and/or microsatellite metastases
Distant metastases (M)
M0: no clinical or radiographic evidence of distant metastases
-
M1: evidence of distant metastases with or without elevation of serum lactate dehydrogenase (LDH) level indicated by (1): LDH elevated or (0): not elevated
-
M1a: distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph node
M1a(0): LDH not elevated
M1a(1): LDH elevated
-
M1b: distant metastasis to lung with or without M1a sites of disease
M1b(0): LDH not elevated
M1b(1): LDH elevated
-
M1c: distant metastasis to non-CNS visceral sites with or without M1a or M1b sites of disease
M1c(0): LDH not elevated
M1c(1): LDH elevated
-
M1d: distant metastasis to CNS with or without M1a, M1b, or M1c sites of disease
M1c(0): LDH not elevated
M1c(1): LDH elevated
-
On this page:
Stage groups
AJCC Prognostic Stage Groups
Stage is defined at different time points in the care of the cancer patient and are based on the continuum of assessment – Clinical (cTNM) – Pathological (pTNM) – Post therapy (ycTNM or ypTNM) – Recurrence (rTNM) and Autopsy (aTNM). A summary of the pathological stage (pTNM) is provided below.
-
stage 0
pTis N0 M0
-
stage IA
pT1a N0 M0
pT1b N0 M0
-
stage IB
pT2a N0 M0
-
stage IIA
pT2b N0 M0
pT3a N0 M0
-
stage IIB
pT3b N0 M0
pT4a N0 M0
-
stage IIC
pT4b N0 M0
-
stage IIIA
pT1a-T2a; N1a/N2a; M0
-
stage IIIB
pT0-T3a; N1a-N2b; M0
-
stage IIIC
pT0-T4b; N2b-N2c; M0
-
stage IIID
pT4b; N3a-N3c; M0
-
stage IV
[Any pT], [Any N], M1a-d
Treatment and prognosis:
Estimated five year survival rates 4:
-
stage I
IA ~ 99%
IB ~ 97%
-
stage II
IIA ~ 94%
IIB ~ 87%
IIC ~ 82%
-
stage III
IIIA ~ 93%
IIIB ~ 83%
IIIC ~ 69%
IIID ~32%
-
stage IV
IV < 10%




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.