Neuroblastomas are tumours of neuroblastic origin. Although they may occur anywhere along the sympathetic chain, the vast majority arise from the adrenal gland.
They represent the most common extracranial solid childhood malignancy and are the third most common childhood tumour after leukaemia and brain malignancies. They account for ~15% of childhood cancer deaths.
On this page:
Epidemiology
The tumours typically occur in infants and very young children (mean age of presentation being ~22 months) with 95% of cases diagnosed before the age of 10 years. Occasionally, they may be identified antenatally or immediately at birth (see congenital neuroblastoma) 2.
Associations
The vast majority of neuroblastomas are sporadic. Sometimes, they may be associated with 1-4:
central failure of ventilation
Clinical presentation
Typically with pain or a palpable mass and abdominal distension, although numerous other presentations may be encountered due to local mass effect.
Other accompanying syndromes include:
Hutchinson syndrome: bone metastases may present with pain or limping and irritability or proptosis with periorbital and cranial bumps 2,7
Pepper syndrome: hepatomegaly due to extensive liver metastasis
blueberry muffin syndrome: multiple cutaneous lesions
opsomyoclonus 5: rapid, involuntary conjugate fast eye movements
proptosis and periorbital ecchymoses ("raccoon eyes"): orbital metastases
Pathology
Location
Neuroblastomas arise from the sympathetic nervous system 2,3, specifically from the primitive neuroectodermal cells or neural crest cells (adrenal medulla precursor).
Intra-abdominal disease (two-thirds of cases) is more prevalent than intrathoracic disease. Specific sites include:
adrenal glands: most common site of origin, 35%
-
retroperitoneum: 30-35%
coeliac axis
paravertebral sympathetic chain
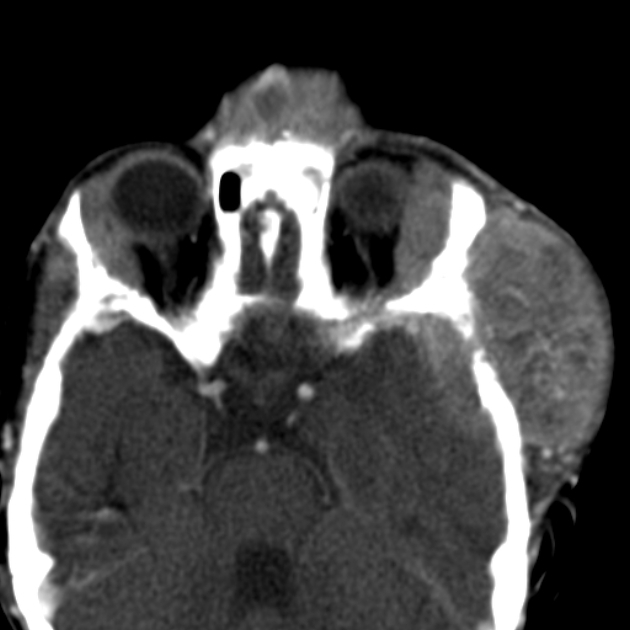
neck: 1-5%
pelvis: 2-3%
Macroscopic appearance
Macroscopically, they tend to be large grey-tan coloured soft lesions with or without a fibrous pseudocapsule. For this reason, some are well-defined, and some are infiltrative. Areas of necrosis, haemorrhage, and particularly calcification are common.
Microscopic appearance
Microscopically, they are similar to small round blue cell tumours 3. They also form Homer Wright rosettes 3.
Markers
Most secrete catecholamines vanillylmandelic acid (VMA) and homovanillic acid (HVA) 2.
Genetics
The majority demonstrate chromosome 1p deletion and N-MYC amplification.
Staging
For staging refer to neuroblastoma staging.
Metastatic disease is common and has a variety of patterns:
-
bone
most common
-
liver
diffuse infiltration (more common in stage 4S)
focal hypoenhancing masses
-
lung and pleura
discrete nodules
diffuse consolidation
pleural disease is uncommon
-
brain and meninges
dural metastases can be diffuse or nodular
brain metastases are uncommon but variable in appearance
Radiographic features
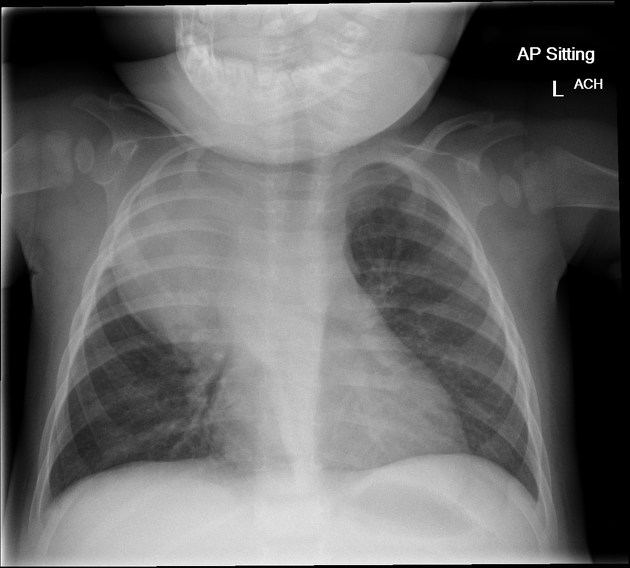
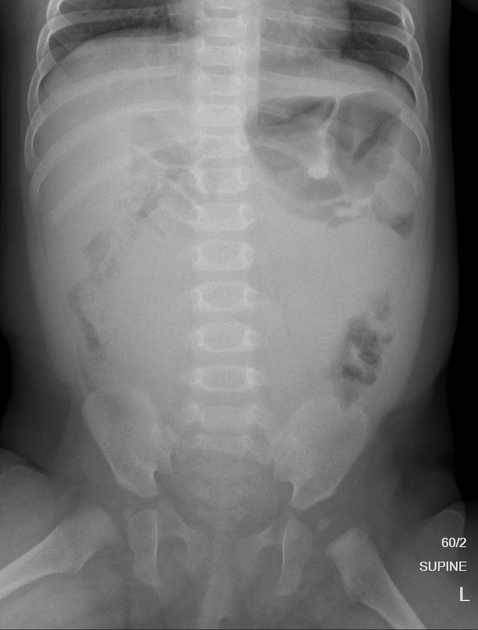
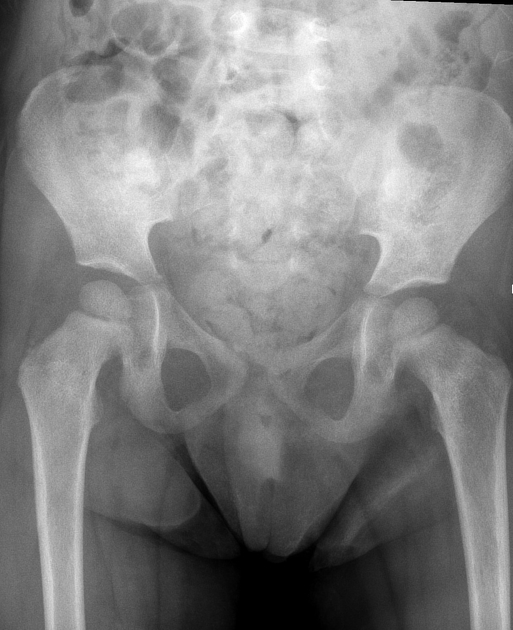
Plain radiograph
Appearances are non-specific, typically demonstrating an intrathoracic soft-tissue mass or an intra-abdominal mass displacing adjacent organs. Pressure on adjacent bones may cause remodelling of ribs, vertebral bodies or pedicle thinning. Up to 30% may have evidence of calcification on the plain film.
Bone metastases are usually ill-defined and lucent (i.e. osteolytic), with periosteal reaction or metaphyseal lucency. Sclerotic bone metastases are uncommon 2.
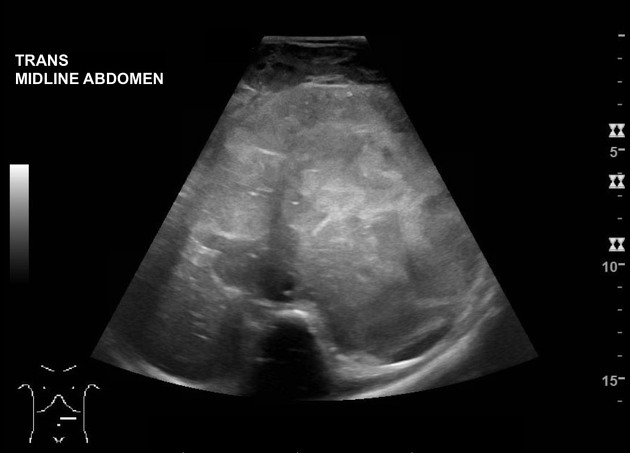
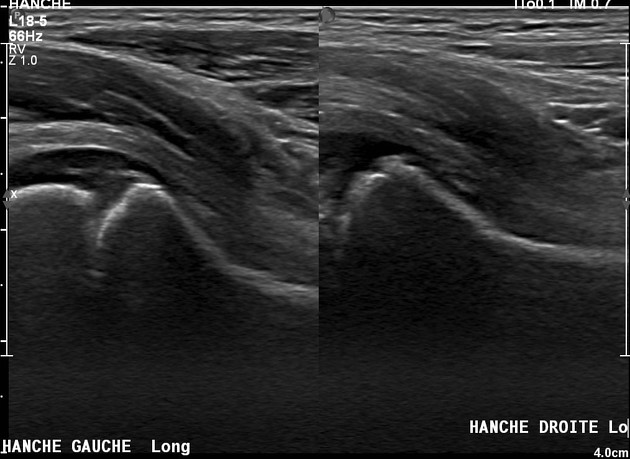
Ultrasound
Neuroblastoma on ultrasound demonstrates a heterogeneous mass with internal vascularity. Often there are areas of necrosis that appear as regions of low echogenicity. Calcification may or may not be evident on ultrasound 2.
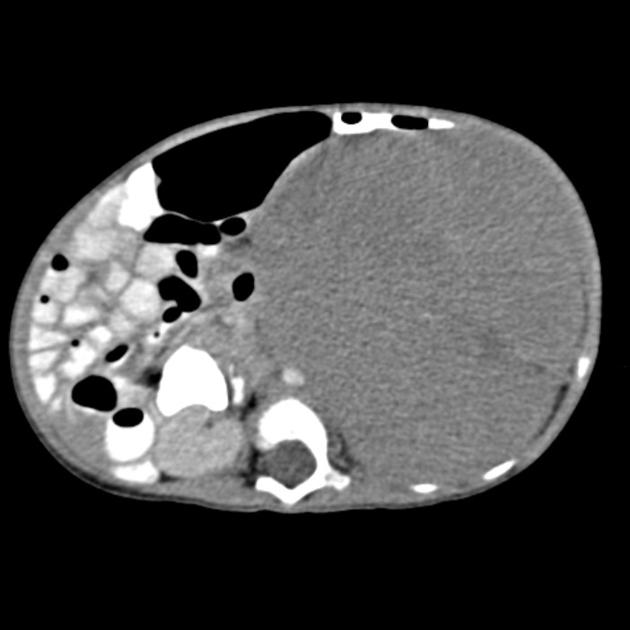
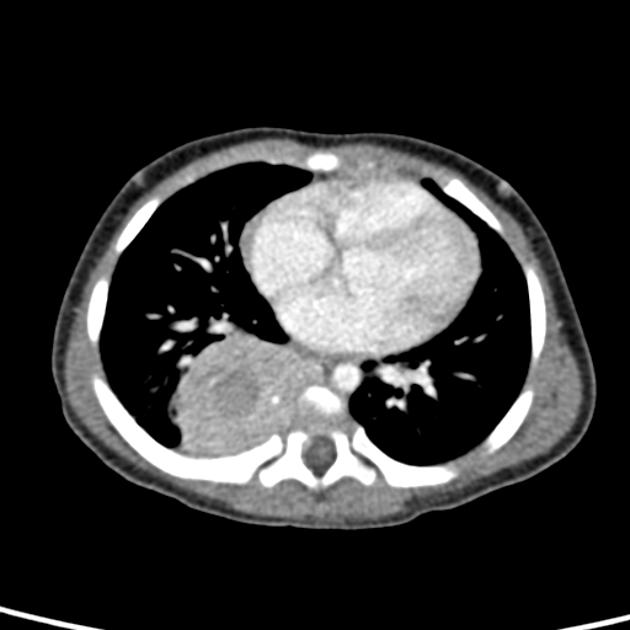
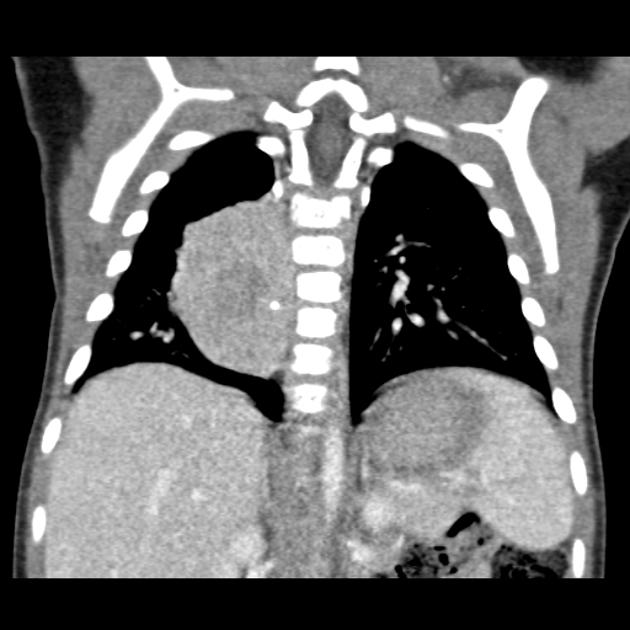
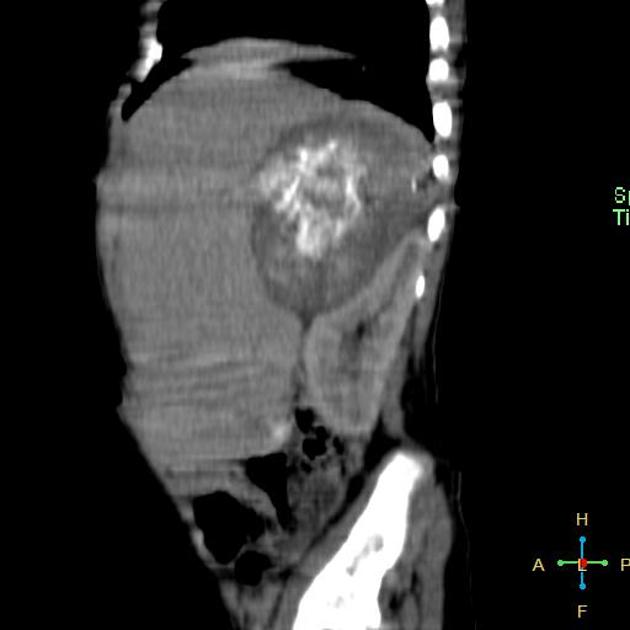
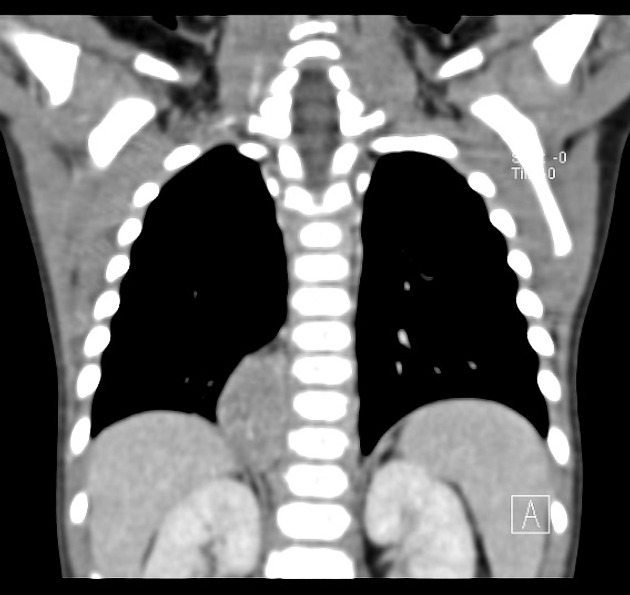
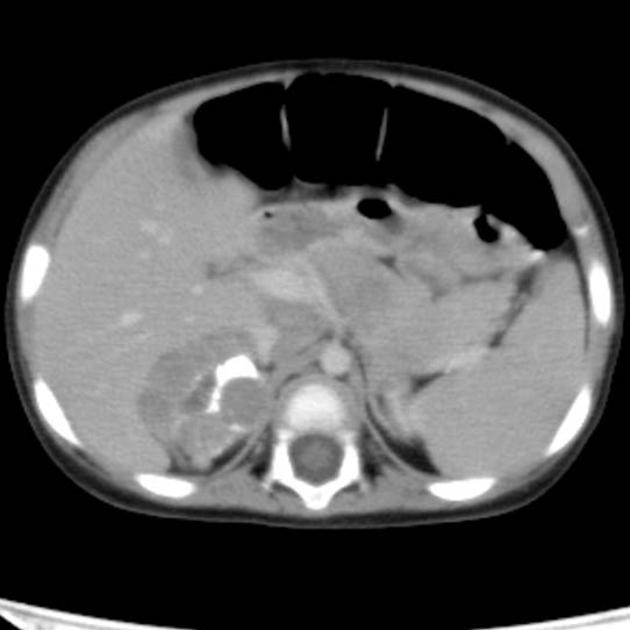
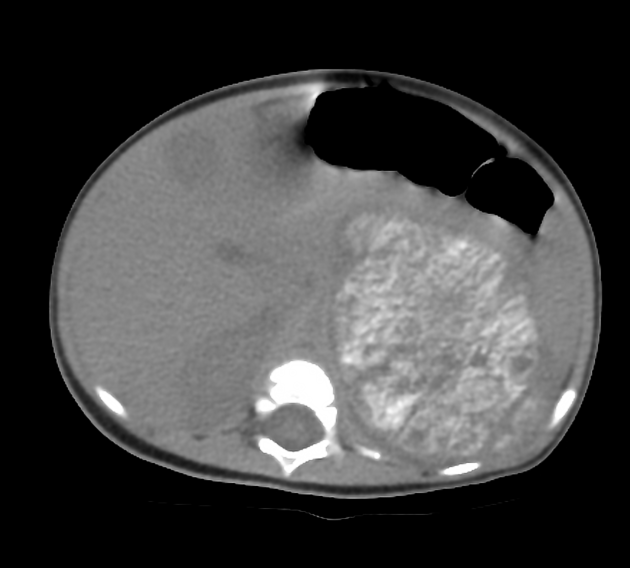
CT
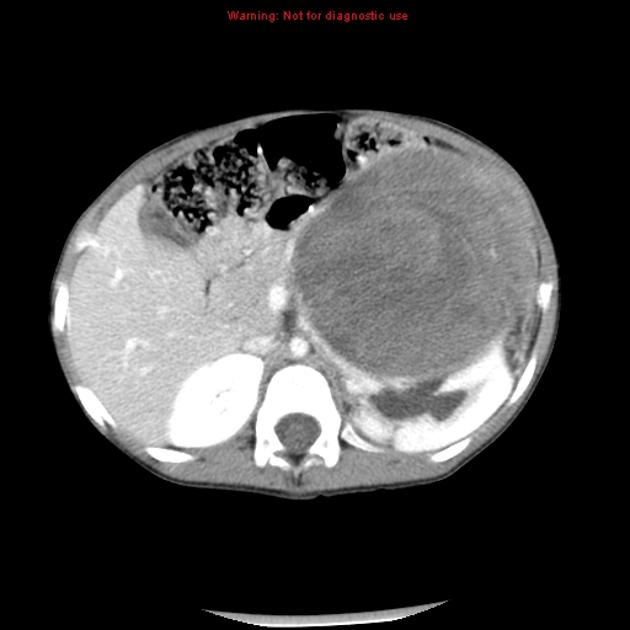
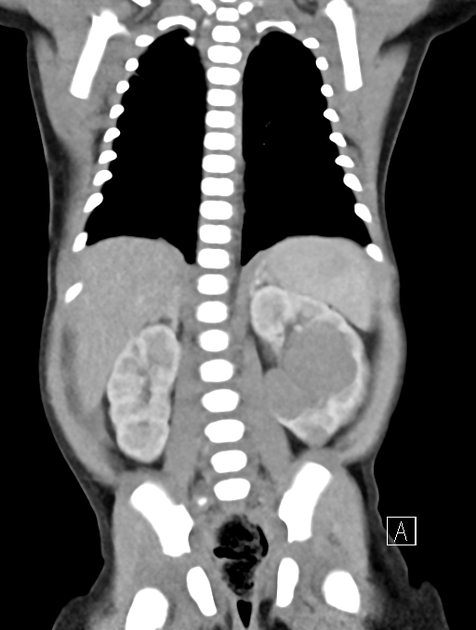
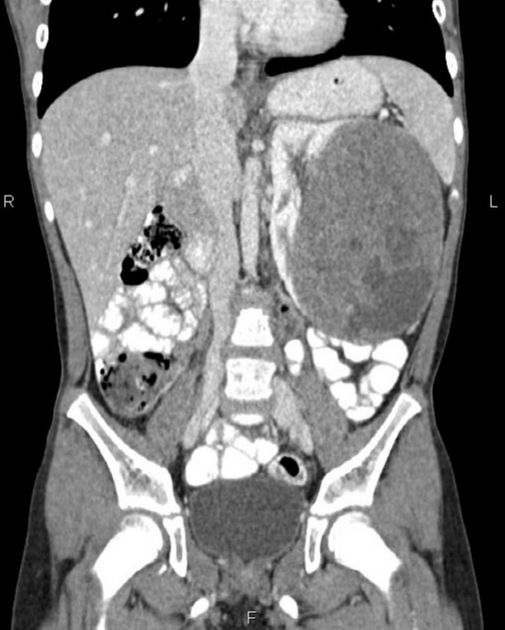
On CT, the tumour typically is heterogeneous with calcifications seen in 80-90% of cases 2. Areas of necrosis are of low attenuation.
The tumour morphology is often helpful, with the mass seen insinuating itself beneath the aorta and lifting it off the vertebral column. It tends to encase vessels and may lead to compression. Adjacent organs are usually displaced, although in more aggressive tumours direct invasion of the psoas muscle or kidney can be seen. The latter can make distinguishing neuroblastoma from Wilms tumour difficult (see neuroblastoma vs Wilms tumour).
Lymph node enlargement is often present.
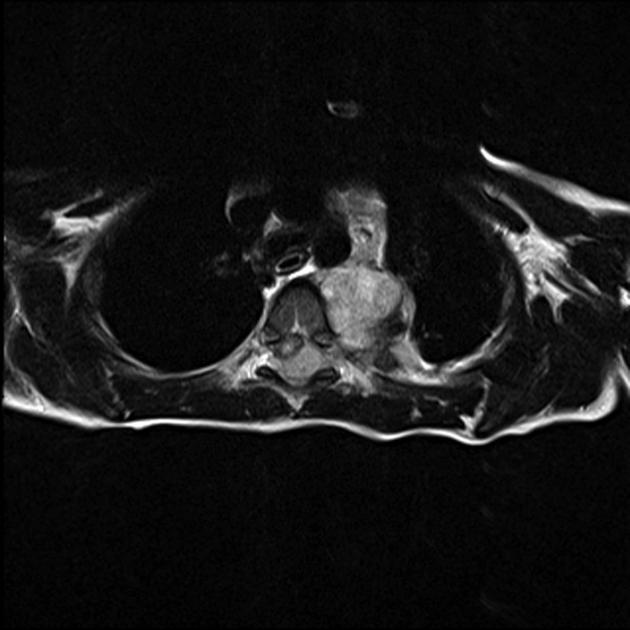
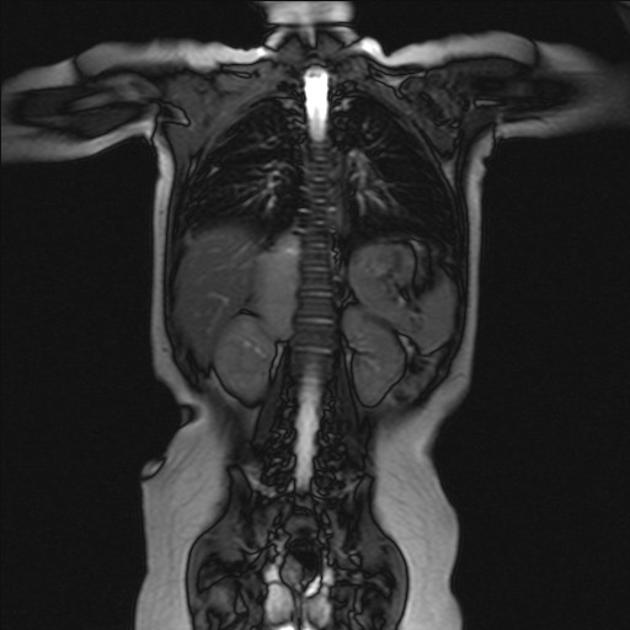
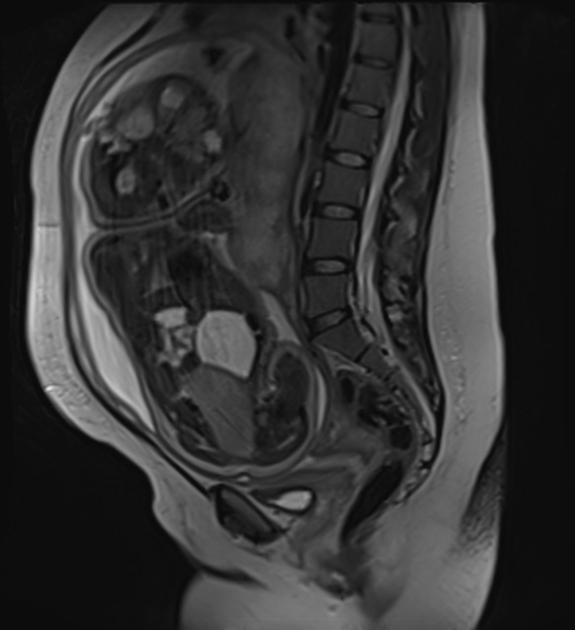
MRI
MRI is superior to all other modalities in assessing: 2
the organ of origin
intracranial or intraspinal disease
bone marrow disease
On MRI:
T1: heterogeneous and iso to hypointense
-
T2
heterogeneous and hyperintense
cystic/necrotic areas have very high intensity
signal voids may be evident
T1 C+ (Gd): variable and heterogeneous enhancement
Nuclear medicine
Some compounds are used for diagnosis and staging:
-
pentetreotide labelled to indium-111 (a somatostatin analogue)
not specific for neuroblastic tissue
-
MIBG (metaiodobenzylguanidine labelled to iodine-123)
95% of neuroblastomas secrete catecholamines, however, 10-30% of neuroblastomas are negative on MIBG
sensitivity: 88%
specificity: 99% (for sympathetic tissue) 2
does not distinguish between neuroblastoma, ganglioneuroblastoma, ganglioneuroma, or neuroendocrine tumours such as phaeochromocytoma
FDG PET-CT
Surveillance for metastatic recurrence:
-
Tc-99m MDP (technetium 99m-methyl diphosphonate)
36% of primary tumours negative
mainly to evaluate bone metastases
also able to detect some lung and liver metastases 2
Treatment and prognosis
Treatment depends on the patient's stage. Localised tumours considered to be low-risk are surgically excised or sent for minimally invasive surgery, and patients tend to do very well (see below). In high-risk tumours, a combination of surgery, chemotherapy +/- bone marrow transplantation is employed, unfortunately with poor overall results. In some cases, where tumours are very large, presurgical chemotherapy to attempt to downstage the tumour may be administered 2.
Patients with stage 1, 2, or 4S have a better prognosis. Unfortunately 40-60% of patients present with stage 3 or 4 diseases 4. For advanced disease, the age of the child is most important 3.
-
<1 year of age: 80-90% 1-year event-free survival
>1 year of age: 50% 3-year survival
-
<1 year of age: 60-75% 1-year event-free survival
>1 year of age: 15% 3-year survival
Poor prognostic factors
later age of onset: >18 months
higher stage: particularly in the presence of metastasis
N-MYC mutation
chromosome 1p deletion
unfavourable Shimada histology index
Better prognostic factors
TRK-A expression
Differential diagnosis
For intrathoracic neuroblastoma consider:
For intra-abdominal neuroblastoma consider:





 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.