Spinal cord stimulators, also known as dorsal column stimulators, are surgically placed devices intended to provide symptom relief in individuals with chronic neurological pain (e.g. failed back syndrome, brachial plexopathy, complex regional pain syndrome).
However, their use remains controversial. A 2023 Cochrane review stated that there is insufficient evidence to support their use outside of a clinical trial setting 3. Others contested this conclusion 4,5.
On this page:
Mechanism of action
Spinal cord stimulators use low-voltage electrical current delivered by electrodes on the surface of the cord to prevent pain signals from reaching the brain. The underlying pathophysiology is not entirely understood; however, an increase in levels of GABA and serotonin stimulated by an electrical pulse in the area of dorsal horns is one potential mechanism 1.
Patients may feel a tingling sensation in the area of electrode placement when the stimulator is on.
Procedure
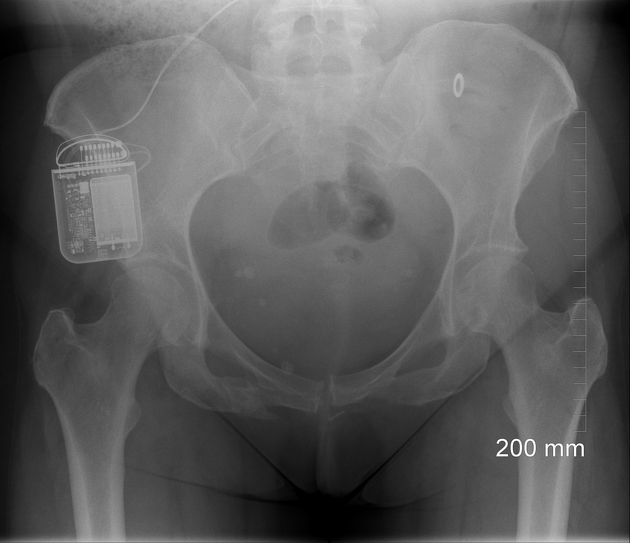
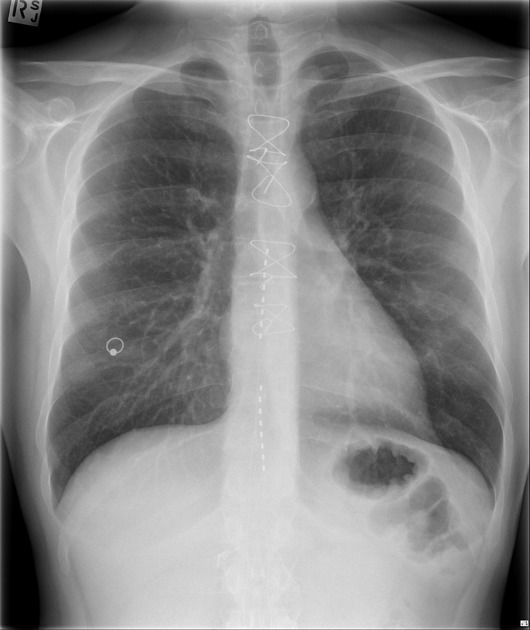
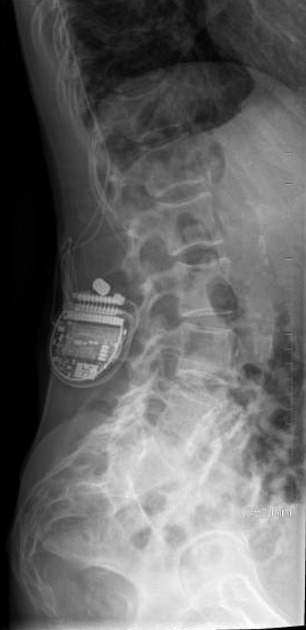
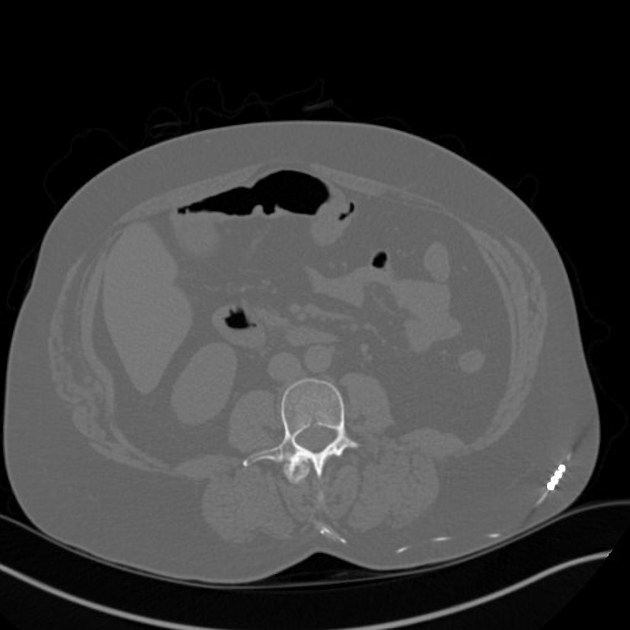
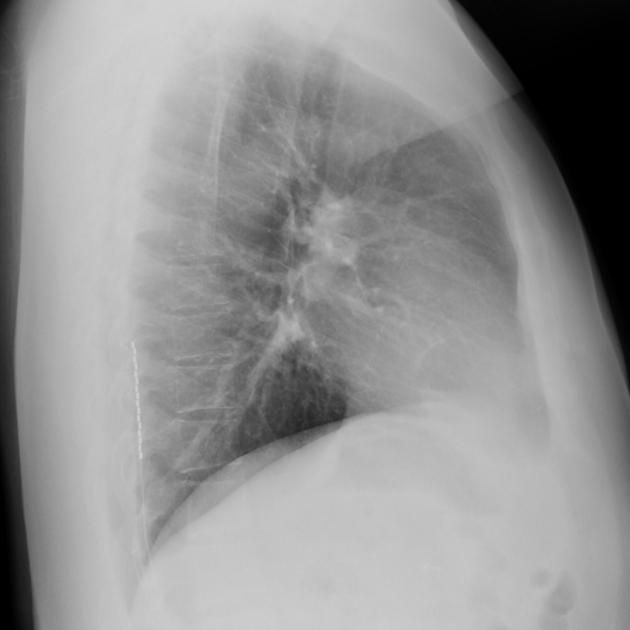
An internal pulse generator is implanted in the subcutaneous tissues of the flank, paraspinal, abdominal or upper gluteal regions, with leads extending into the spinal canal with epidural electrodes placed adjacent to the dorsal columns. Two types of electrodes can be implanted 2:
cylindrical: placed via percutaneous technique under fluoroscopy
paddle: placed surgically via laminectomy or laminotomy
Complications
Complications can be serious and include 2:
device-related, e.g. device malfunction, lead/electrode break or migration
biological, e.g. infection, fluid collection or wound dehiscence, CSF leak, cord/nerve damage, epidural hematoma/abscess




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.