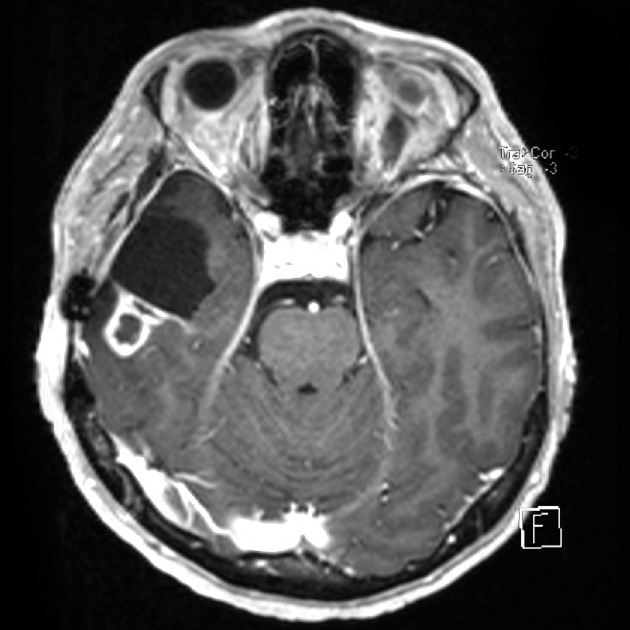
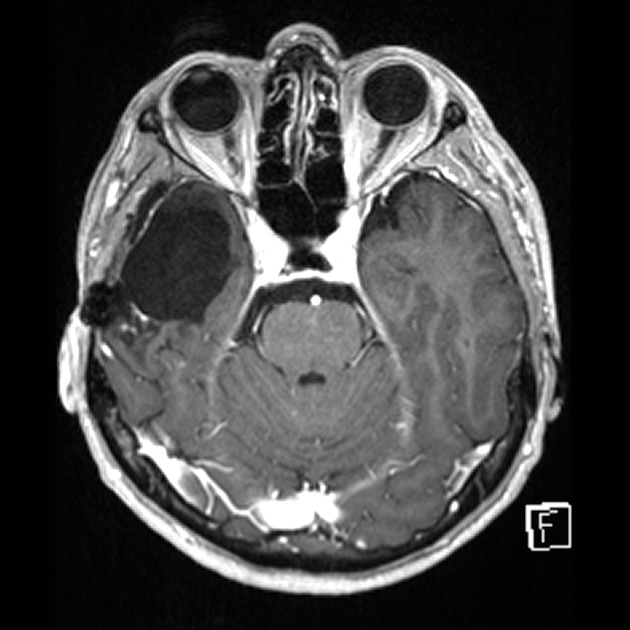
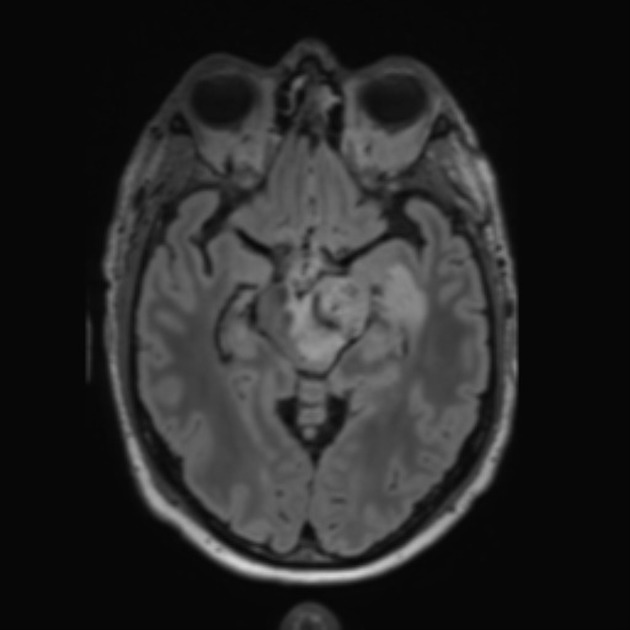
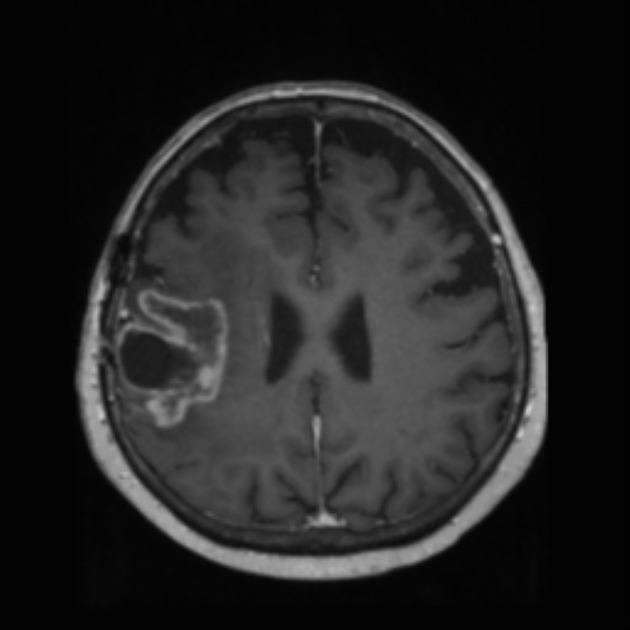
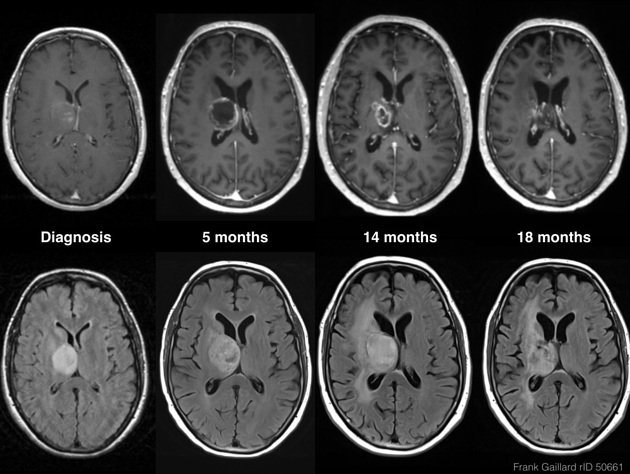
Tumor pseudoprogression, also known simply as pseudoprogression, is a relatively common finding in brain tumor imaging follow-up, especially for high-grade gliomas (e.g. glioblastoma).
On this page:
Terminology
Due to an overlap between the definitions of pseudoprogression and radiation necrosis, it is not incorrect to say that pseudoprogression represents a mild and self-limiting variant of treatment-related necrosis 1,2.
Epidemiology
It is observed in ~30% of patients after combined chemotherapy and radiotherapy (Stupp protocol). Radiotherapy alone is less likely to result in pseudoprogression, only observed in ~15% of patients.
Risk factors
Tumors with methylated MGMT show pseudoprogression more frequently, mainly when treated with temozolomide 1,5,8.
Clinical presentation
In almost 60% of cases, pseudoprogression occurs within the first three months after completing treatment, but it may occur from the first few weeks to 6 months after treatment 1-3. Generally patients remain clinically stable without the clinical deterioration usually seen with rapid tumor progression 1.
Pathology
Pseudoprogression is related to endothelial damage and consequent tissue hypoxia observed after treatment and it has an early occurrence (~60%), usually in the first 3 months after the treatment, but it may occur from the first few weeks to 6 months after treatment ref.
Brain post-radiation treatment effects can be divided into pseudoprogression and radiation necrosis 4. Importantly, the likelihood of developing pseudoprogression is strongly influenced by the radiation dose. It is unlikely to occur in brain that has received <60 Gy 9-11.
Radiographic features
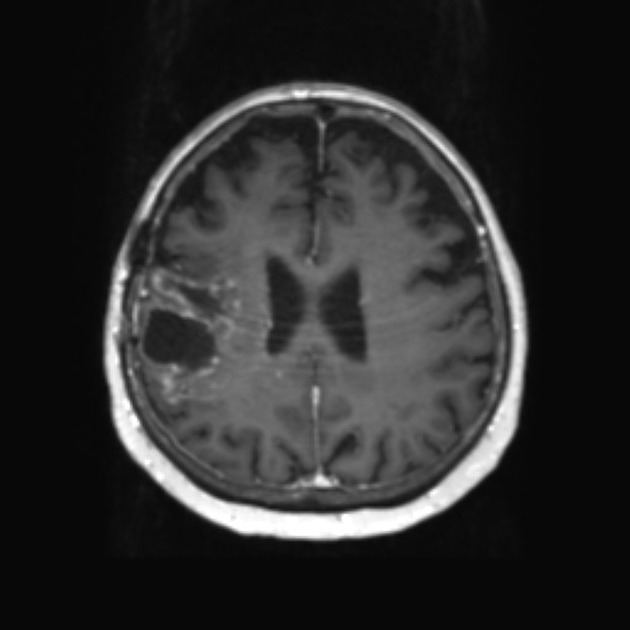
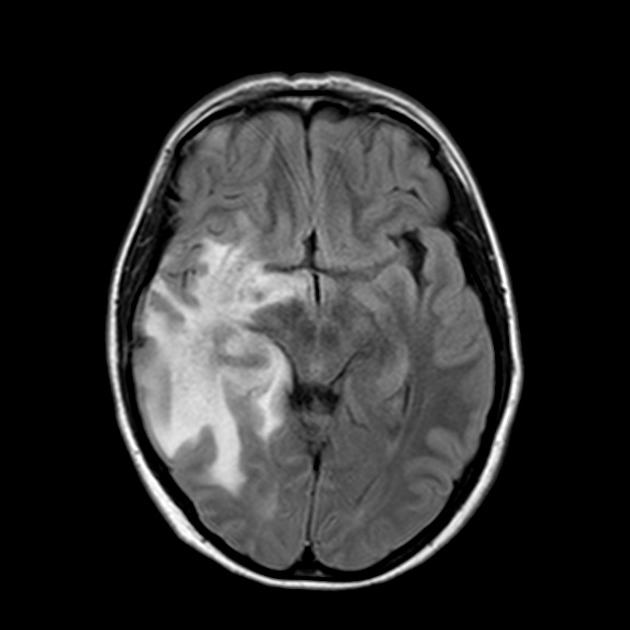
The hallmark of pseudoprogression is increased size of the enhancing component of a glioblastoma, and this has proven challenging in the trial setting as the most widely used criteria (Macdonald criteria and RANO criteria) struggle to distinguish between pseudoprogression and true disease progression as they largely rely on only the size of the enhancing component 6. As such conventional CT and MRI are insensitive to the distinction. However, advanced sequences (MR spectroscopy, perfusion, ADC values) are of significant help.
MRI
A good quality MRI including advanced sequences is essential (see MRI protocol: brain tumor) along with access to prior imaging and radiation field maps. Key features of pseudoprogression include:
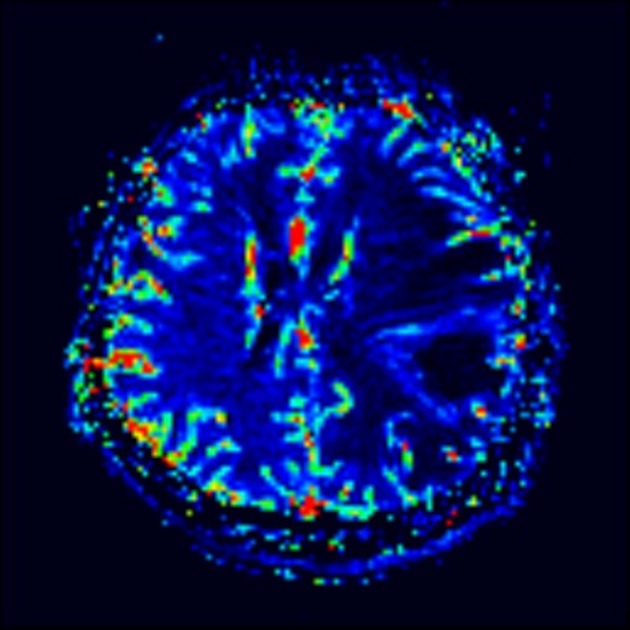
perfusion: reduced cerebral blood volume (viable tumor will usually have increased rCBV) 6
-
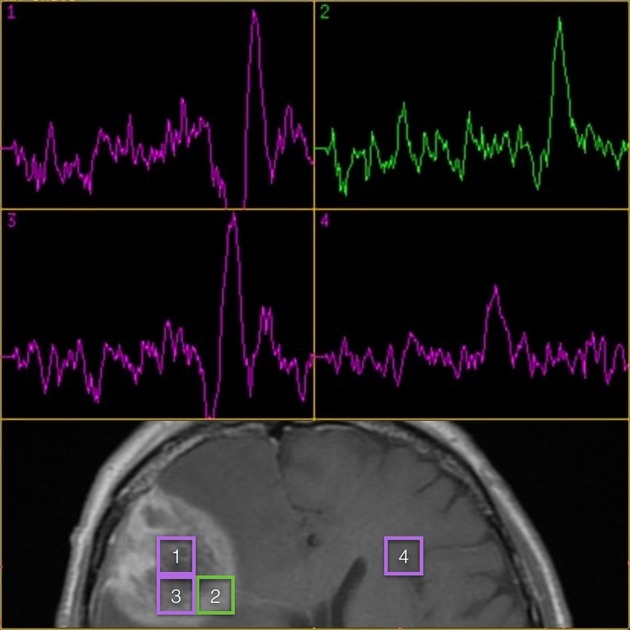
spectroscopy 7
low choline
choline/NAA ratio ≤1.4 8
increased lactate peak
increased lipid peak
the trace may also be generally flat (hypometabolic)
-
ADC
tumors that respond to treatment and result in pseudoprogression will have elevated ADC values due to cell death
ADC mean values ≥1300 x 10-6 mm2/s 8
Treatment and prognosis
Not only does pseudoprogression not represent disease progression, it often is a marker of longer survival, presumably because it represents a robust response to treatment 8.
Differential diagnosis
The primary differential diagnosis is that of true tumor progression. Unfortunately, no single feature is pathognomonic of tumor versus pseudoprogression, however, the following features generally favor tumor:
location: outside the 60 Gy field
timing: late occurrence e.g. >12 months after Stupp protocol
perfusion: elevated cerebral blood volume
diffusion: lower ADC values
spectroscopy: high choline, low lipid and lactate
clinical: patient doing poorly
histology: MGMT unmethylated






 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.