Epstein-Barr virus-associated smooth muscle tumors are rare tumors found throughout the body encountered in immunocompromised individuals.
On this page:
Epidemiology
These tumors are generally exceedingly rare and only seen with any frequency in the setting of immunosuppression, particularly in HIV/AIDS patients, but also post-transplantation and in common variable immunodeficiency syndrome.
In the HIV population, they are encountered as non-AIDS-defining cancers in both adult and pediatric populations 1. The age range is therefore defined by the underlying cause of immunosuppression, but they tend to be encountered in young adults (mean age of diagnosis 25 years; range: 2.7 to 49 years) 1. No strong gender predilection has been identified 1.
Clinical presentation
Clinical presentation is primarily the result of mass effect on adjacent structures 5 and will, therefore, vary widely depending on location (see below).
Pathology
EBV-SMT range from indolent leiomyomas to aggressive leiomyosarcomas 1,5. In both cases, co-infection with Ebstein-Barr virus is necessary for diagnosis 1.
Histology
EBV-SMT are characterized by interlacing fascicles of mildly to moderately pleomorphic atypical spindle cells with plentiful eosinophilic cytoplasm. Presence of necrosis, cellularity and mitotic count will vary.
Location
Unlike sporadic smooth muscle tumors, which tend to occur where there is an abundance of smooth muscle cells, EBV-SMT occur in atypical locations that are normally largely free of smooth muscle 3.
Although they occur almost anywhere in the body, the central nervous system (both intra- and extra-axial) is the most common site of involvement, with the gastrointestinal tract, liver, skin, lungs, pharynx, and larynx also relatively frequently involved 1-3.
In approximately half of cases, far more frequently than sporadic smooth muscle tumors, EBV-SMT are multifocal at the time of diagnosis, representing simultaneous multiple primaries rather than metastases 1-3.
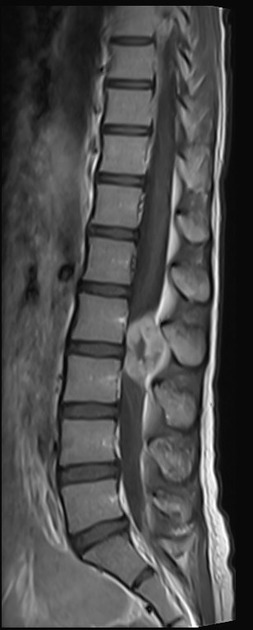
Radiographic features
Imaging findings are non-specific, with tumors appearing as well circumscribed enhancing soft-tissue masses 1,3.
When arising in the central nervous system, EBV-SMTs are most frequently extra-axial dural lesions, most closely resembling meningiomas 3-5.
CT
Hyperdense well circumscribed masses with vivid contrast enhancement 4.
MRI
T1: intermediate signal, similar to muscle or grey matter
T1 C+: vivid usually solid contrast enhancement 3
T2: low signal 3
-
SWI
calcium usually absent
necrosis and hemorrhage variable 3
DWI/ADC: no diffusion restriction 4
perfusion: low rCBV 3
Treatment and prognosis
Treatment of EBV-SMT is primarily with resection, although radiotherapy and chemotherapy have also been used. In the setting of HIV/AIDS, antiretroviral therapy (ART) is also potentially indicated 1.
Additional treatment with EBV-specific immunotherapy, demethylating agents and mTOR/AKT inhibitors are being investigated 5.
Prognosis and aggressiveness are variable but seem to be somewhat more favorable than conventional 'sporadic' leiomyosarcomas 1,5.
Differential diagnosis
The differential diagnosis will depend on the location 3-5.
-
intracranial
meningioma or other dural pathology
Tolosa-Hunt syndrome when involving cavernous sinus
infections (cryptococcoma, tuberculoma, syphilitic gumma etc..)
-
spinal
meningioma
-
elsewhere, other soft tissue tumors
neurogenic tumors
sarcomas (e.g. Kaposi sarcoma)




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.