Idiopathic intracranial hypertension (IIH), also known as pseudotumour cerebri, is a syndrome with signs and symptoms of increased intracranial pressure but where a causative mass or hydrocephalus is not identified.
On this page:
Terminology
The older term benign intracranial hypertension is generally frowned upon due to the fact that some patients with idiopathic intracranial hypertension have a fairly aggressive clinical picture with rapid visual loss.
Interestingly, as it has become evident that at least some patients present with IIH due to identifiable transverse sinus stenosis, some authors now advocate reverting to the older term pseudotumour cerebri as in these patients the condition is possibly not idiopathic 15. An alternative approach is to move these patients into a group termed secondary intracranial hypertension 15.
Epidemiology
By far the most commonly affected demographic is middle-aged obese females, affected approximately ten times more frequently than other patient groups 34. The aetiological link between being female, overweight and developing idiopathic intracranial hypertension remains to be elucidated.
Obesity is encountered in the majority of cases 34, and as the prevalence of obesity is increasing, so too is the incidence of this diagnosis 31.
Less commonly IIH can also be encountered in males, usually older and less likely to be obese 15. It is rare in the paediatric population, being more common in the 12-17 year age group than in the 2-12 year age group 15,29.
Associations
A variety of conditions are known to be associated with idiopathic intracranial hypertension including:
-
endocrine
excessive thyroxine replacement in children
-
drugs
tetracyclines, such as doxycycline 2
growth hormone
hypervitaminosis A from dietary intake or other retinoids, such as all-trans retinoic acid, isotretinoin, or retinol
Diagnosis
Modified Dandy criteria
The modified Dandy criteria were revised to establish the diagnosis in the Idiopathic Intracranial Hypertension Treatment Trial 24:
presence of signs and symptoms of increased intracranial pressure
absence of localising findings on neurologic exam except those known to occur from increased intracranial pressure
absence of deformity, displacement, or obstruction of the ventricular system and otherwise normal neurodiagnostic studies, except for evidence of increased CSF pressure*; abnormal neuroimaging except for empty sella turcica, optic nerve sheath with filled out CSF spaces, and smooth-walled non-flow-related venous sinus stenosis or collapse should lead to another diagnosis
awake and alert patient
no other cause of increased intracranial pressure present
*The opening CSF pressure should be either >25.0 cm H2O or 20.0-25.0 cm H2O with at least one of the following additional findings:
Frisen grade II papilloedema
echography negative for drusen or other disc anomalies mimicking disc oedema (pseudopapilledema)
lateral sinus stenosis or collapse
partially empty sella and optic nerve sheaths with filled out CSF spaces
Revised Friedman criteria
A competing set of diagnostic criteria were proposed in 2013 and are also commonly used 37. The original terminology was for pseudotumour cerebri syndrome but the term idiopathic intracranial hypertension has since supplanted it.
The criteria place patients into one of four diagnostic subgroups:
definite idiopathic intracranial hypertension: opening pressure ≥25 cm CSF (H2O) and papilloedema
probable idiopathic intracranial hypertension: opening pressure <25 cm CSF and papilloedema
definite idiopathic intracranial hypertension without papilloedema: opening pressure ≥25 cm CSF and abducens nerve palsy
suggested idiopathic intracranial hypertension without papilloedema: opening pressure ≥25 cm CSF and ≥3 out of 4 neuroimaging signs (empty sella, flattening of the posterior aspect of the globe, distension of the perioptic subarachnoid space, or transverse venous sinus stenosis)
These subgroups have several additional common requirements:
neurologic examination: normal except for cranial nerve abnormalities
neuroimaging: no hydrocephalus, mass, structural lesion, or abnormal meningeal enhancement on MRI (without and with contrast) and, if not female or obese, MRV (if MRI is unavailable or contraindicated, contrast-enhanced CT may be used)
laboratory: normal CSF composition
For children, the threshold opening pressure is 28 cm CSF rather than 25 cm CSF, unless the child is not sedated and not obese.
Clinical presentation
Patients usually present with headaches, visual problems (transient or gradual visual loss), pulse-synchronous tinnitus, photopsia, and/or eye pain 15,31.
Papilloedema is the hallmark finding on fundoscopic examination, which is typically bilateral but uncommonly may be unilateral or even absent, making the clinical diagnosis difficult 6. Neurological examination is usually normal, except visual field deficit or sixth cranial nerve palsy are sometimes encountered.
Lumbar puncture is central to diagnosis. The CSF composition is normal but the opening pressure is elevated (with 20-25 cm H2O considered equivocal and >25 cm H2O considered definitely abnormal). It is controversial whether positioning during lumbar puncture is clinically important, with some insisting that lateral decubitus is the most accurate but others believing the default position for fluoroscopy-guided lumbar puncture, prone, is close enough 25. It should also be noted that opening pressure can vary during the day. One study continuously measuring CSF pressures demonstrated many patients had intermittent pressure waves with amplitudes of 50–80 mmHg (68–109 cm H2O) that lasted 5 to 20 minutes 26.
Aberrant arachnoid granulations, also referred to as meningoceles, can result in secondary CSF leaks that can present as rhinorrhoea, otorrhoea, intracranial hypotension, and recurrent bacterial meningitis 7,9. In such patients it is often only after dural repair that intracranial hypertension becomes evident; presumably, the CSF leak from the meningocele normalised pressure 9.
Pathology
The pathogenesis is poorly understood. Various mechanisms have been proposed, including decreased CSF absorption, increased CSF production, increased intravascular volume, increased intracranial venous pressure, hormonal changes, altered aquaporin-4 channels and abnormality in function of the glymphatic system 1,15,32-34.
Venous sinus stenosis is increasingly recognised as an important factor although whether it is the primary inciting abnormality or a potentiating factor remains to be fully established. The increasingly established clinical efficacy of venous stenting suggests that it is, however, not merely a biomarker 31. It has also been shown that the pressure within the torcula or the dural venous sinuses and the opening pressure measured at lumbar puncture are very closely correlated 31.
A study also found an association between decreased glymphatic clearance and papilloedema in patients with IIH 32. Whether this is a primary driving cause or also the sequela of impaired venous outflow remains to be determined 32-34.
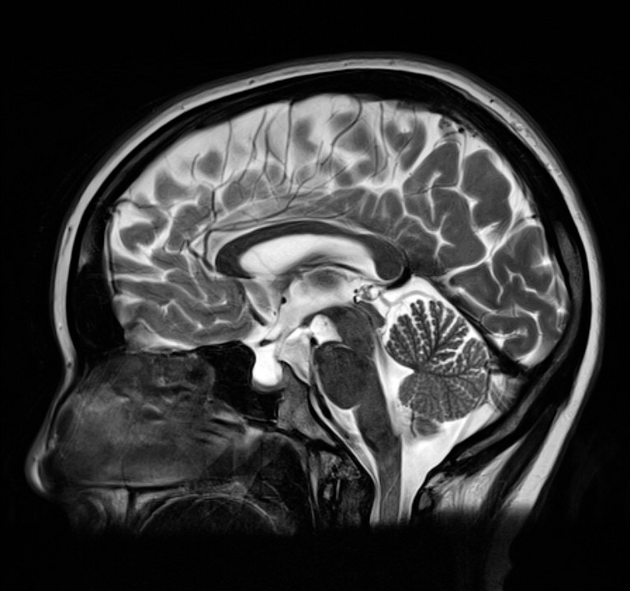
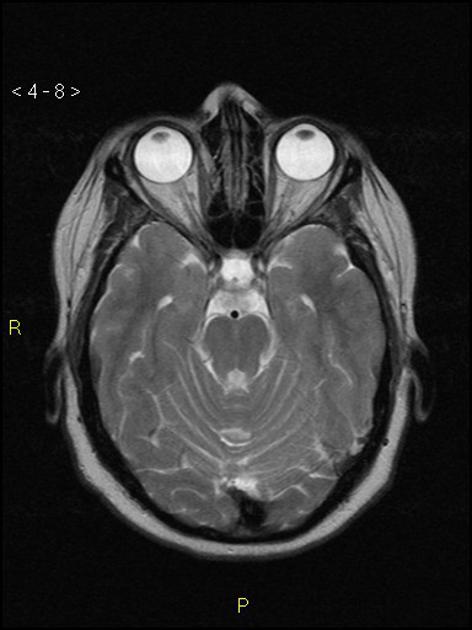
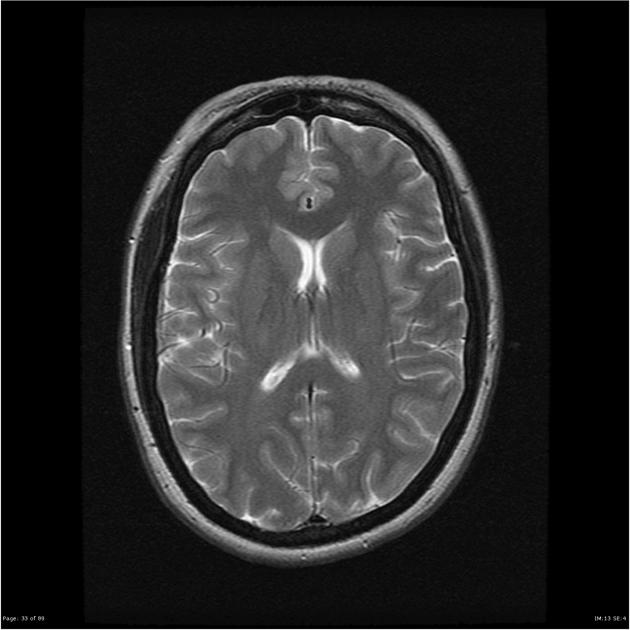
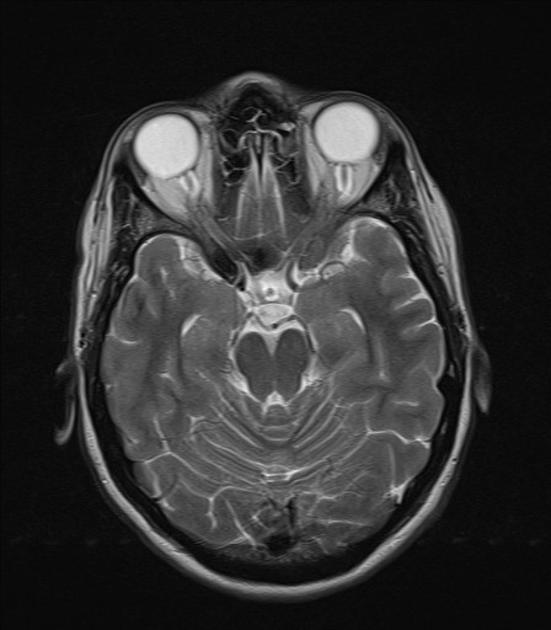
Radiographic features
CT/MRI
Imaging of the brain with CT or MRI without and with contrast, and possibly CT or MR venography, is essential in patients with suspected idiopathic intracranial hypertension to exclude elevated CSF pressure due to other causes such as brain tumour, dural sinus thrombosis, hydrocephalus, etc.
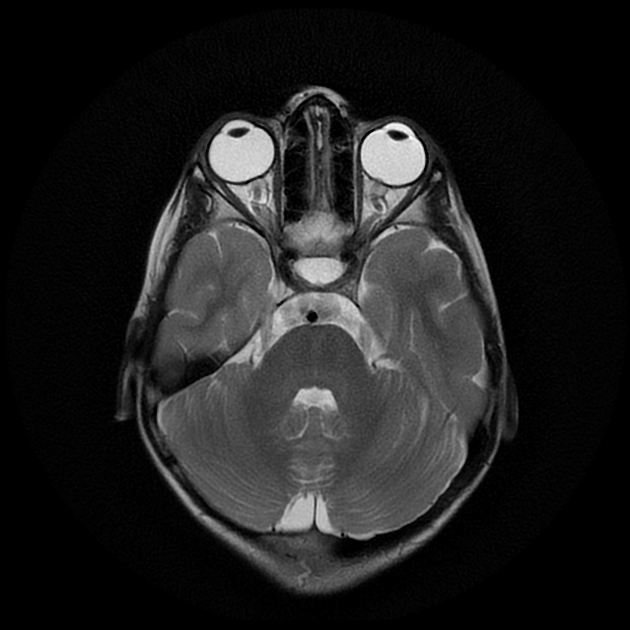
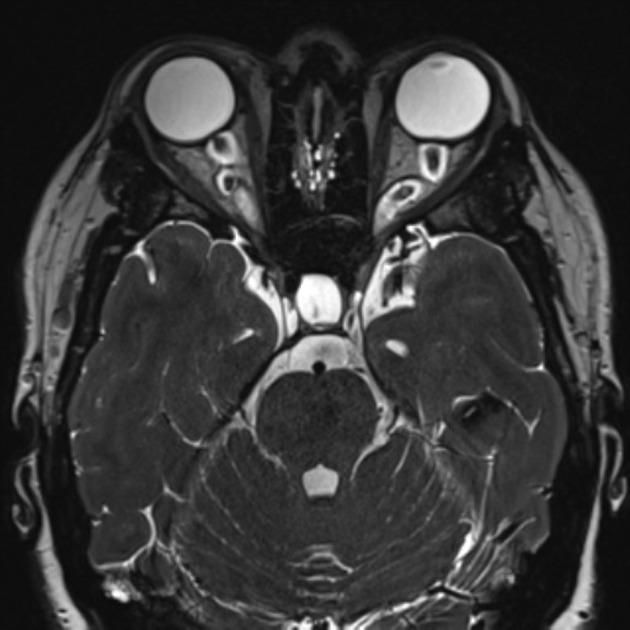
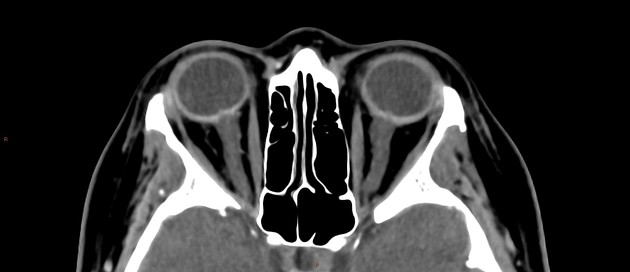
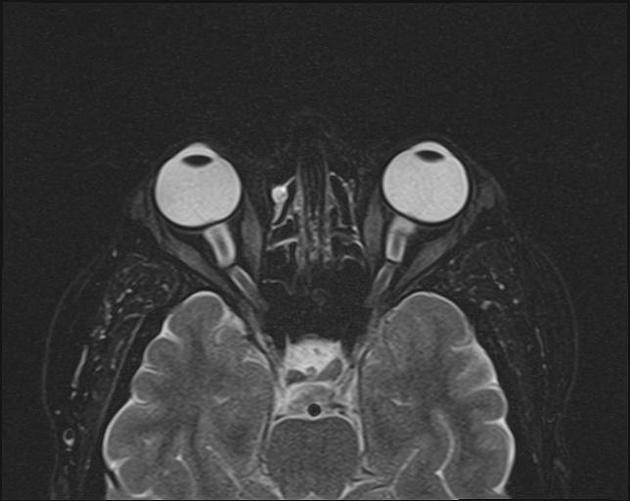
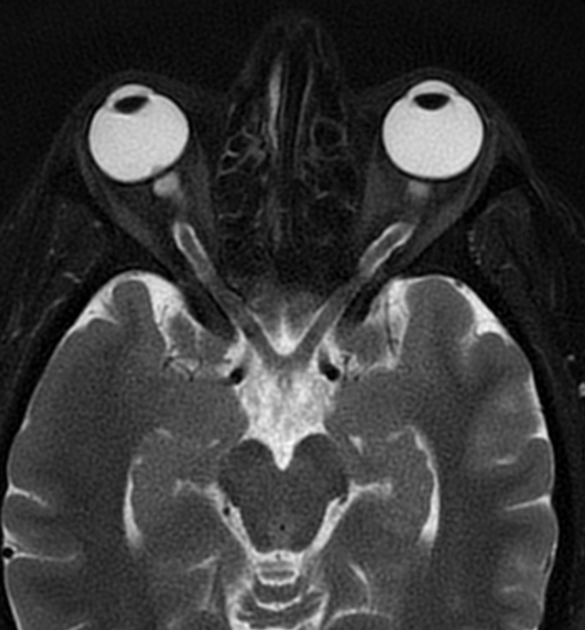
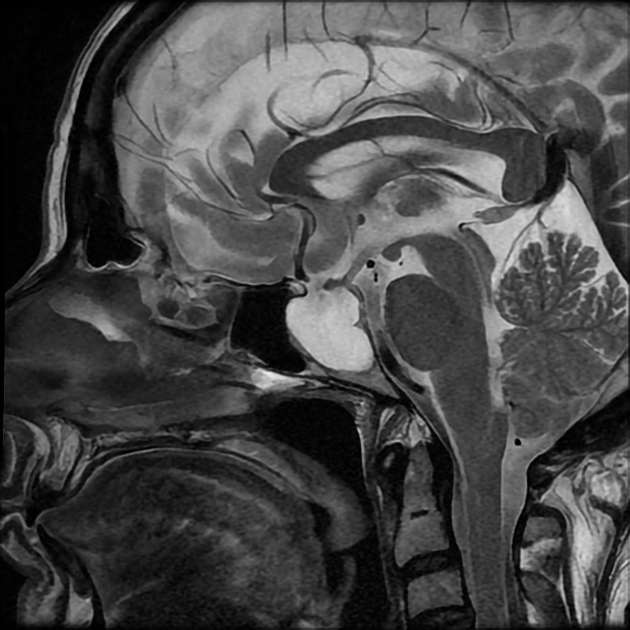
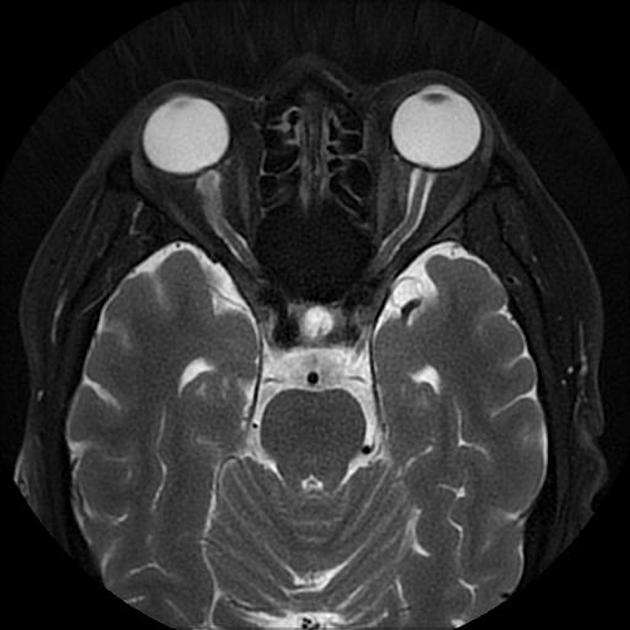
In the absence of a cause for intracranial hypertension, imaging features that support the diagnosis of idiopathic intracranial hypertension include 5,6-9,15,23:
-
orbits
-
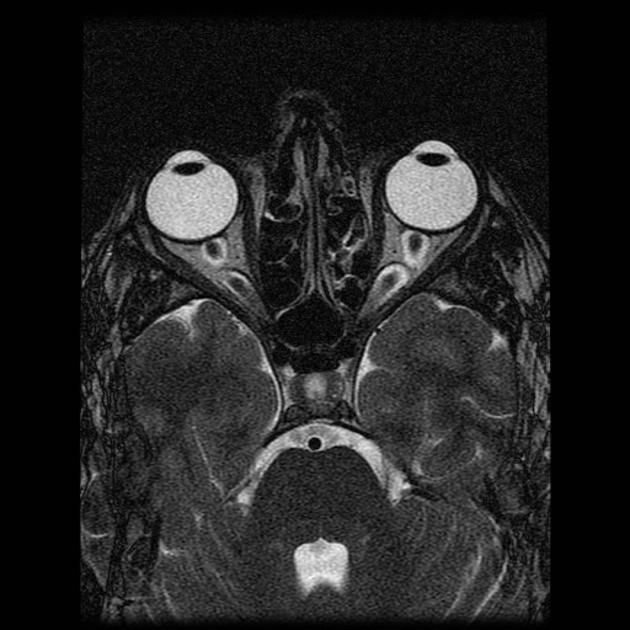
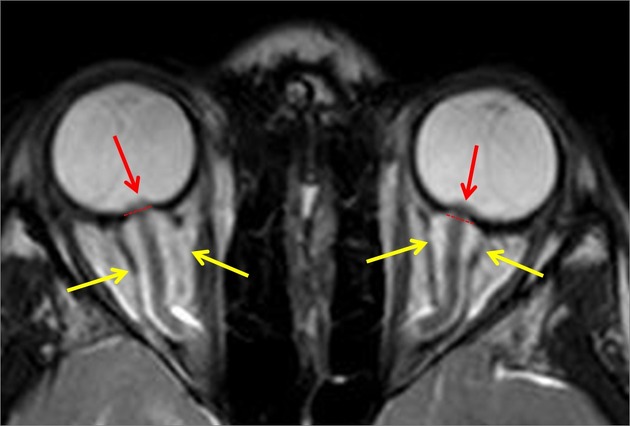
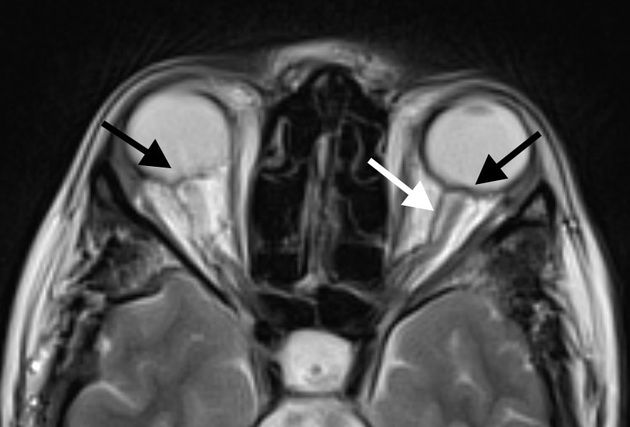
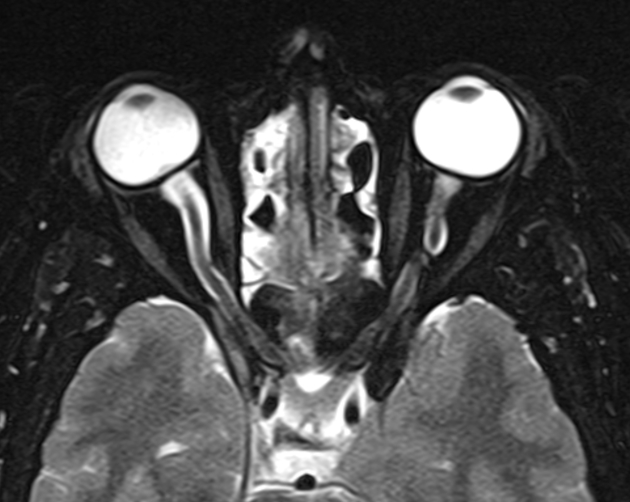
optic nerve sheath distension (70%, more sensitively/reliably detected by high-resolution 3D T2-weighted imaging) 23,40,41,43
optic nerve sheath diameter >5.3-6 mm or subarachnoid space >2 mm measured 3 mm posterior to the globe on axial or coronal images ref
a small series (c. 2024) showed greater accuracy in predicting IIH using the "arachnoid bulk ratio" but this requires further validation 48
posterior globe flattening (60%)
optic nerve tortuosity in vertical or horizontal planes (40%)
papilloedema/optic nerve head protrusion (30%)
optic nerve head enhancement (~ 45%, range10-80%; more sensitively detected by contrast-enhanced 3D T2-weighted FLAIR) 36
-
-
enlarged arachnoid outpouchings
-
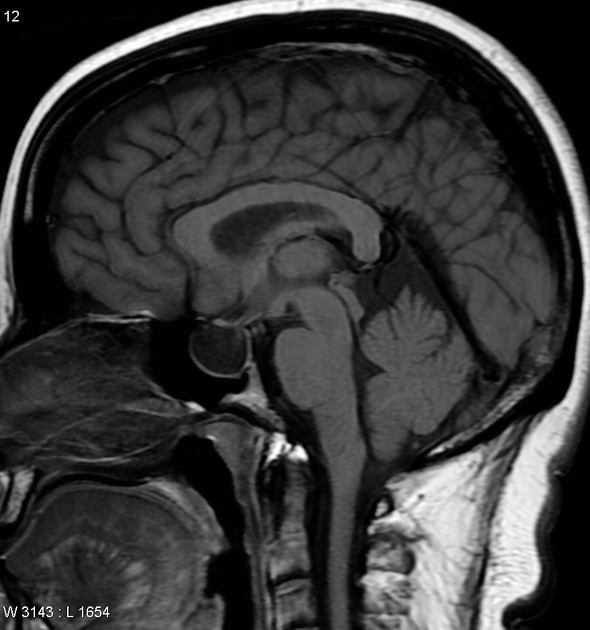
partially empty sella turcica (60%)
pituitary occupies less than two-thirds of the pituitary fossa (at least pituitary height loss grade III) 38 but some use a lower threshold of 50% 23
pituitary gland height <4.8 mm 43,44
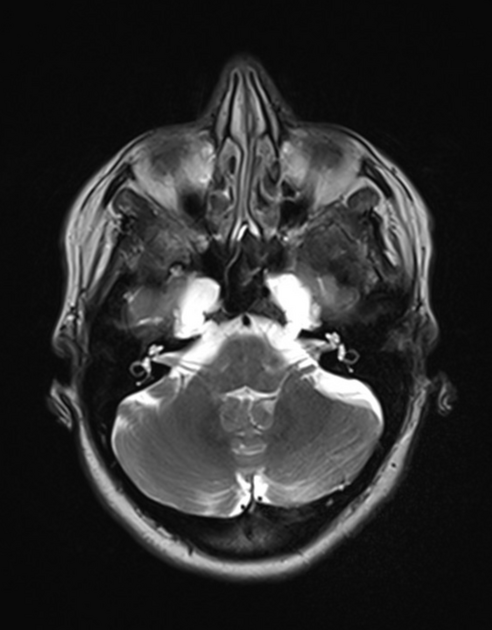
Meckel cave enlargement 9,18 (but sometimes it is narrowed 39)
arachnoid pits (aberrant arachnoid granulations)/small meningoceles, typically within the temporal bone and sphenoid wing 9
enlarged oculomotor cistern (CSF space around the oculomotor nerve in the lateral wall of the cavernous sinus) 18
prominent perivascular spaces 30
-
-
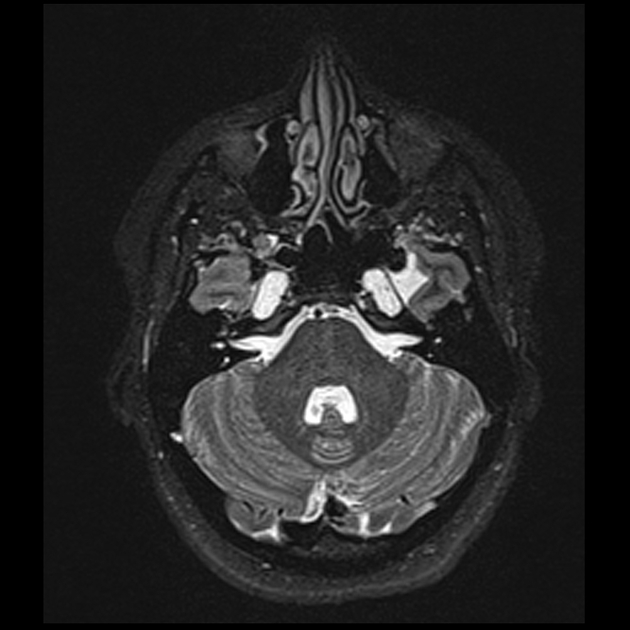
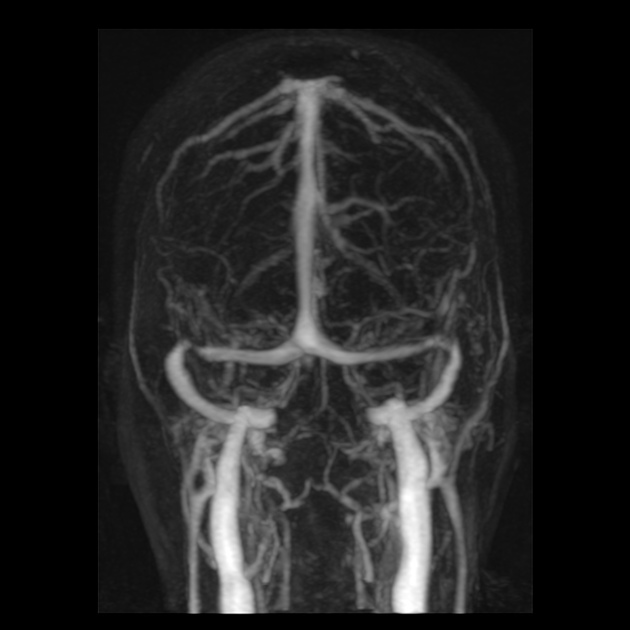
venous outflow obstruction
-
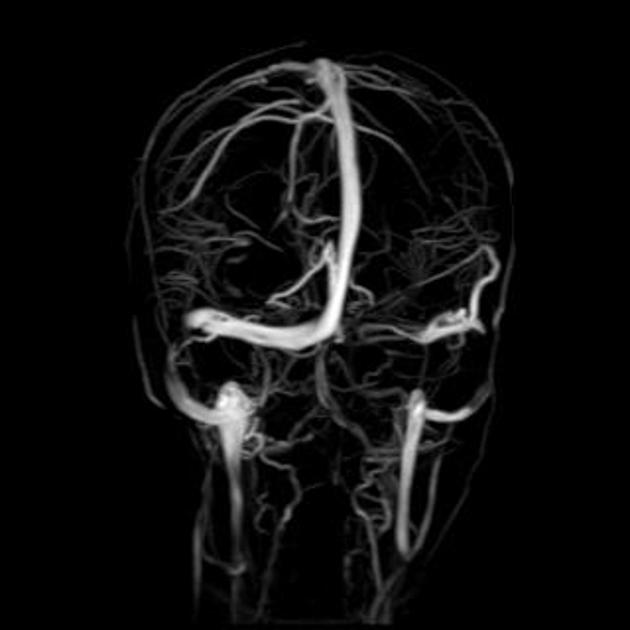
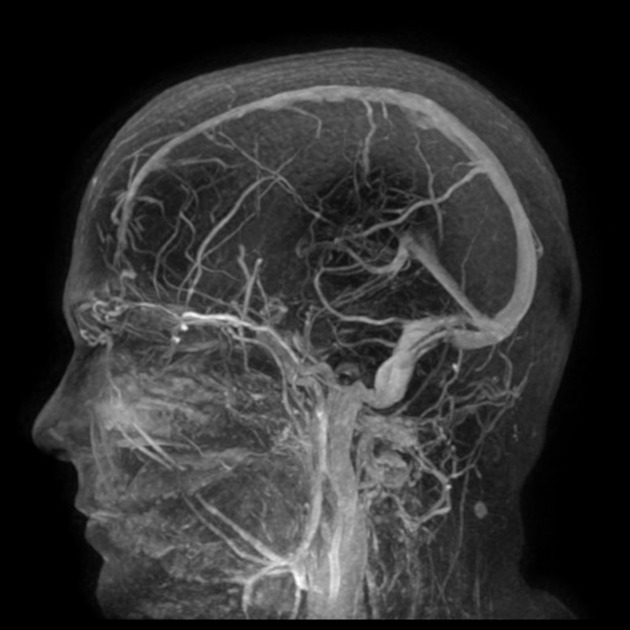
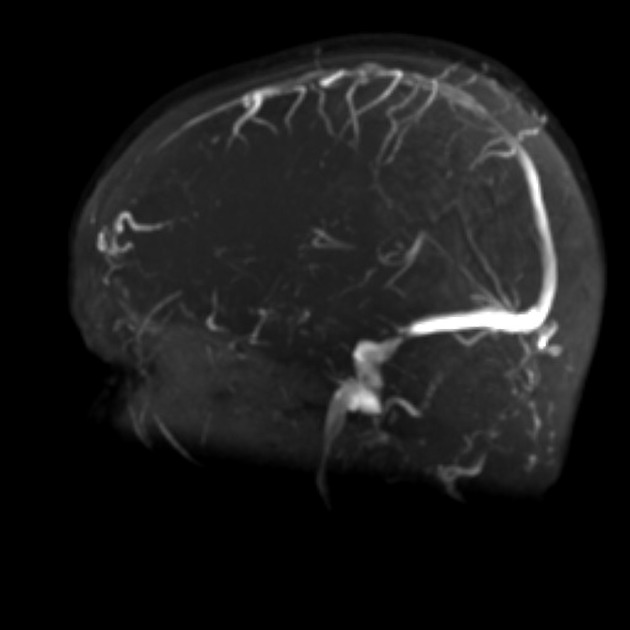
transverse sinus stenosis (80%) 23,31
significant bilateral transverse sinus narrowing due to any combination of arachnoid granulations (focal, most commonly at the lateral aspect near the transverse-sigmoid sinus junction), extrinsic compression (segmental, which can be relieved after CSF withdrawal 11,12), or hypoplasia/aplasia (diffuse), not related to current or remote thrombosis 8
index of transverse sinus stenosis ≥4: the index is the product of stenosis grades on the left and right sides, where 0 = normal, 1 = stenosis up to one-third (<33%) compared to the immediate pre-stenotic segment, 2 = stenosis between one-third and two-thirds (33-66%), 3 = stenosis more than two-thirds (>66%), and 4 = hypoplasia defined as full-length transverse sinus diameter less than one-third of the superior sagittal sinus 5,28,42
combined venous conduit patency score ≤4: the score is the sum of patency grades on the left and right transverse-sigmoid conduit, where 4 = normal (75-100% of the diameter of the distal superior sagittal sinus), 3 = mild stenosis (50-75% patent), 2 = moderate stenosis (25-50% patent), 1 = severe stenosis or hypoplasia (<25% patent), and 0 = absent (discontinuity or aplastic) 45,46
internal jugular vein stenosis (including styloidogenic jugular venous compression) 47
-
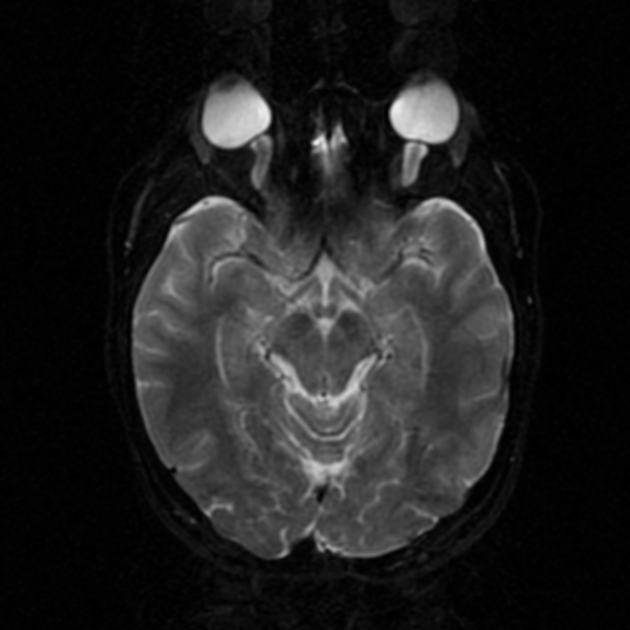
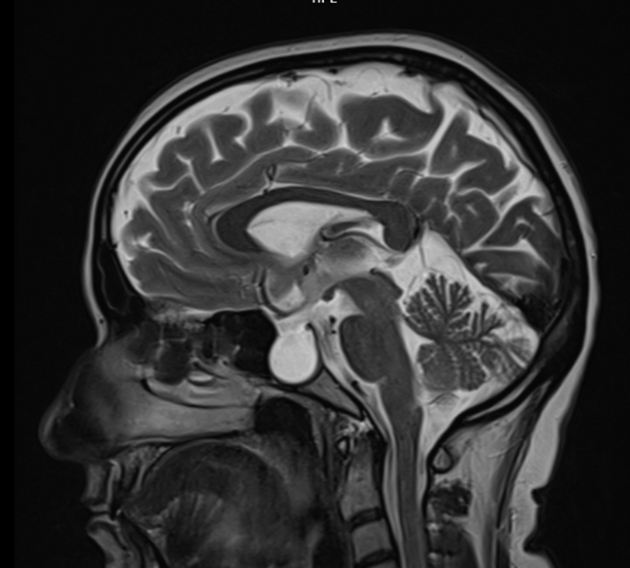
acquired cerebellar tonsillar ectopia (20%) 16,23
slit-like ventricles (15%) 15,23
increased subcutaneous fat thickness in the scalp and neck (a slim patient is unlikely to develop idiopathic intracranial hypertension) 17
Although bony changes are permanent, the rest may be reversible with treatment 3,11,12.
It is important to note that some of these findings in isolation may be normal (such as partially empty sella, particularly in older patients). Optimal diagnostic accuracy requires taking into account the entire constellation of multiple imaging findings as well clinical features.
Angiography (DSA)
In addition to enabling venous stenting, catheter venography allows for venous manometry to be performed to evaluate transverse sinus stenosis 31. Serial measurements of pressure from the superior sagittal sinus down to the internal jugular vein and right atrium allows for the detection of a focal pressure differential across of stenosis (so-called trans-stenosis gradient) 31.
Treatment and prognosis
First-line treatment options include 13,31:
-
weight loss in patients with a BMI >30 kg/m2
weight loss of ~15% is possibly curative
-
carbonic anhydrase inhibitors
acetazolamide
topiramate
Invasive treatment options, usually reserved for refractory cases, include 13:
-
venous sinus stenting for transverse sinus stenosis
typically reserved for severe cases with a trans-stenotic gradient of >8 mmHg 31
-
increasingly shown to be effective 4,10,14,31,35
~80% improved headache 14,35, ~95% improved tinnitus 14,35, ~90% improved papilloedema 14,35, ~90% improved visual symptoms 35
reduction in opening pressure by ~15 cmH2O 35
treatment failure ~13% 14,35
-
internal jugular venous decompression
less well established
relies on either stenting or removal of compressing structure (e.g. styloidectomy, mastoid process, muscles, masses, etc.) 31
bariatric surgery as a surgical weight loss strategy
optic nerve sheath fenestration (only if vision is acutely threatened)
serial CSF letting or CSF shunting (e.g. ventriculoperitoneal shunt, lumboperitoneal shunt)
History and etymology
Idiopathic intracranial hypertension was first reported in 1893 by Heinrich Quincke, and termed "meningitis serosa". The term "pseudotumour cerebri" was later introduced in 1904, and later still "benign intracranial hypertension" in 1955 (not to be confused with benign intracranial hypotension) 15.
Differential diagnosis
Other causes of intracranial hypertension and papilloedema should be sought, such as intracranial mass or hydrocephalus. Even without mass effect, venous obstruction (e.g. venous sinus thrombosis) and leptomeningeal diseases (e.g. meningitis) can mimic the intracranial findings. Therefore, CT or MR venography and lumbar puncture are part of the usual workup of suspected idiopathic intracranial hypertension.
Additionally, in patients with prominent cerebellar tonsillar ectopia, the possibility that all findings are in fact due to a Chiari I malformation should be considered, particularly as there is substantial overlap in the demographics and clinical presentation of the two patient groups 16,19. It has even been suggested that some cases of symptomatic intracranial hypertension are secondary to a Chiari I malformation 20. Importantly, however, every attempt should be made to distinguish between the two entities as treatment is different and symptom relief for patients with idiopathic intracranial hypertension with posterior fossa decompression is insignificant 21.






 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.