Isocitrate dehydrogenase (IDH) is an enzyme located within the cytoplasm, peroxisomes and mitochondria. Mutations of the IDH genes are important in a variety of diseases. They form the basis of the classification of diffuse adult-type gliomas in the WHO classification of brain tumors 7 with emerging therapeutic implications 13. Somatic mutations of IDH result in enchondromatosis syndromes: Ollier disease and Maffucci syndrome 8.
On this page:
Function
Normal function
Isocitrate dehydrogenases are enzymes that catalyze the oxidative decarboxylation of isocitrate to 2-oxoglutarate (also known as α-ketoglutarate) which occurs within mitochondria, cytoplasm and peroxisomes. This reaction also produces NADPH (IDH1 and IDH2) or NADH (IDH3) 4,5.
In mitochondria, isocitrate dehydrogenase (IDH3) acts at the rate-limiting step of the tricarboxylic acid cycle and results in the production of NADH.
Implications of abnormal function
When IDH1 and 2 are mutated they no longer catalyze the formation of 2-oxoglutarate but rather they gain the ability to catalyze the formation of 2-hydroxyglutarate which accumulates (and can in principle be detected using MRS; see below). This compound bears a structural resemblance to glutamate, a key excitatory neurotransmitter in the human brain. Consequently, it has the capability to stimulate N-methyl-D-aspartate (NMDA) receptors. This phenomenon heightens the susceptibility to seizures, elucidating the prevalent occurrence of seizures in diffuse gliomas with IDH mutations, such as astrocytomas and oligodendrogliomas.
Genetics
A number of genes have been identified that code for isoforms of these enzymes, with IDH1 and IDH2 being most relevant in glioma classification 8.
-
IDH1
located on the long arm of chromosome 2 (2q32)
encodes for cytosolic isocitrate dehydrogenase
mutations affect a single amino acid residue 132, in most instances (>85%) resulting in arginine being replaced with histidine and thus denoted R132H 8
responsible for production NADPH
-
IDH2
located on the long arm of chromosome 15 (15q21)
encodes for mitochondrial isocitrate dehydrogenase
mutations affect a single amino acid residue 172, analogous to the R132 residue in IDH1 8
responsible for the production NADPH
-
IDH3
unlike IDH1 and IDH2, which are single genes, IDH3 is a multi-subunit enzyme complex composed of several different subunits
located in mitochondria and part of the citric acid cycle
essential for the production of NADH
Terminology
The terminology can be confusing because although mutation of IDH is seen early in gliomagenesis and is oncogenic it confers a better prognosis than gliomas without the mutation (wildtype) 3. In other words:
IDH-wildtype = IDH negative = no mutation = poor prognosis
IDH-mutant = IDH positive = mutation present = better prognosis
IDH-mutant
Tumors that have mutations of IDH genes are referred to as "IDH-mutant" or in older literature "IDH positive". By definition, essentially all low-grade adult-type diffuse gliomas (astrocytomas and oligodendrogliomas) are IDH-mutant 12. It is important to note that most IDH-mutant tumors are also MGMT-methylated (>80%) 9,11.
IDH-wildtype
As of the 5th edition (2021) of the WHO classification, all glioblastomas are now, by definition, IDH-wildtype 12.
Detection
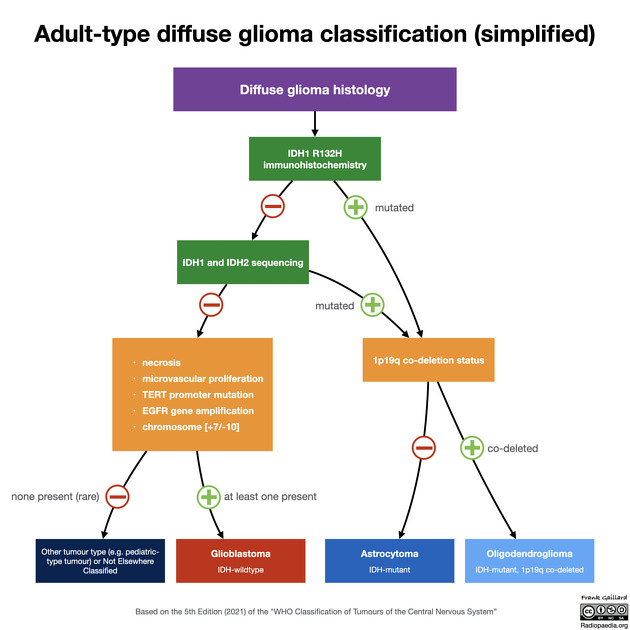
In most instances, IDH status is obtained by performing immunohistochemistry on surgical biopsy specimens. The majority (90%) of IDH mutations in gliomas affect IDH1 with a single amino acid missense mutation at arginine(R)132 replaced by histidine (H); thus denoted as IDH1 R132H. This is the mutation generally tested by immunohistochemistry 7.
If no immunohistochemical reactivity is detected, it is likely but not certain that the tumor is IDH-wildtype. This can only be established with formal genotyping, e.g. using pyrosequencing 3,7. In practice, however, this is not always done, both because it is expensive and in some patient groups (e.g. elderly patients with tumors demonstrating necrosis) it is almost always negative (i.e. these are almost invariably glioblastoma).
Although no single absolute age cut-off exists, in individuals over the age of 55 years with a diagnosis of glioma that is IDH1 R132H negative on immunohistochemistry, the chances of an IDH mutation being detected by next-generation sequencing is low and not considered mandatory 12.
MGMT methylation status is also potentially useful in further reducing the chances that an immunohistochemical IDH1 R132H negative tumor actually harbors a less common mutation. Although the literature is heterogeneous, generally IDH mutated (IDH1 and IDH2) tumors are more likely to also have MGMT methylation (80% for IDH-mutant compared to 60% of IDH-wildtype tumors) 9-11.
Not yet in widespread clinical use, but of tremendous interest, is the assessment of 2-hydroxyglutarate in vivo with MR spectroscopy 3. In tumors with mutated IDH, levels of 2-hydroxyglutarate are elevated, which resonates at 2.25 ppm 3,6.
Therapeutic implications
A number of IDH inhibitors have been investigated as potential therapeutic agents. Vorasidenib, an IDH1 and IDH2 inhibitor that is administered orally and can penetrate the blood brain barrier, has been shown to significantly prolong progression-free survival (27.7 months vs 11.1 months) 13.




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.