Medulloblastomas are the most common malignant brain tumours of childhood, most often presenting as midline masses in the roof of the 4th ventricle (at the superior medullary velum) with associated mass-effect and hydrocephalus. Treatment typically consists of surgical resection, radiation therapy, and chemotherapy, with the prognosis strongly influenced by surgical resection, the presence of CSF metastases at the time of diagnosis, molecular and histological features and expression of the c-erbB-2 (HER2/neu) oncogene.
On this page:
Terminology
Although medulloblastoma has been classically thought of as a single entity, it is becoming increasingly evident that there are many distinct molecular and histological subgroups with overlapping clinical, histological and imaging features 8.
In the 2016 edition of the WHO classification of CNS tumours, four molecular groups were recognised (WNT, SHH, group 3 and group 4).
In the 5th edition (2021) of the WHO classification of CNS tumours, new subgroups were acknowledged based on DNA-methylation profiling and/or transcriptome profiling 17. Additionally, the four histopathological types described in the 2016 edition were combined into one section that describes them as morphologic patterns of one tumour group: medulloblastomas, histologically defined 23.
-
medulloblastoma, SHH-activated
TP53-wildtype
TP53-mutant
subgroups 1-4
-
non-WNT/non-SHH, further divided into:
group 3 and group 4
subgroups 1-8
medulloblastoma, histologically defined
Epidemiology
Overall medulloblastomas, are the most common malignant brain tumour* and account for 12-25% of all paediatric CNS tumours, and 30-40% of paediatric posterior fossa tumours 1,7,17-20.
* Note: in CBTRUS statistical reports "gliomas, malignant NOS" are more common, however, they include a variety of incompletely classified tumours 19,20.
They are also seen in adults but only account for 0.4-1.0% of adult brain tumours 1. Since there are many more adults than children, 14-30% of all medulloblastomas are found in adults.
Taken as a group, there is a moderate male predilection with a M:F ratio of 2:1, although this is only true of group 3 and 4 tumours 8.
They usually present in childhood with 77% of cases before the age of 19. Peak incidence is between 3 and 7 years of age 17. When diagnosed in adulthood, they typically present in the 3rd and 4th decades and are more likely to arise in atypical locations (see below). When they present in adulthood, there is often a better prognosis.
Importantly the age of presentation and gender ratio is influenced by tumour genomics 7-9,12,17:
-
WNT (~10%)
children and adults (not seen in infancy)
M:F 1:2
-
SHH-activated TP53-wildtype (~20%)
infants and adults (rare in children)
M:F 1:1
-
SHH-activated TP53-mutant (~10%)
children
M:F 3:1
-
group 3 (~25%)
infants and children (rare in adults)
M:F 2:1
-
group 4 (~35%)
typically children but encountered in all age groups
M:F 3:1
Associations
Medulloblastomas are associated with a number of syndromes, including:
L-2-hydroxyglutaric aciduria
Clinical presentation
The growth of these very cellular tumours is often rapid and accounts for their relatively rapid clinical onset. Typically, presentation occurs over a few weeks with features that are dominated by symptoms of raised intracranial pressure as a result of obstructive hydrocephalus 7.
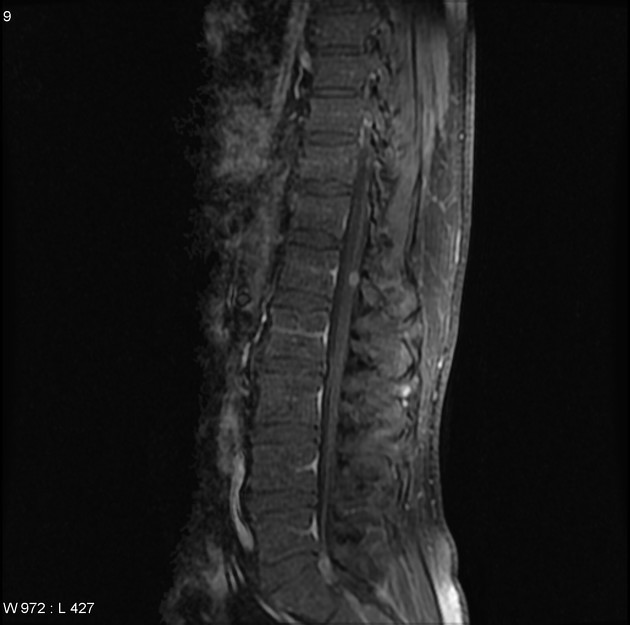
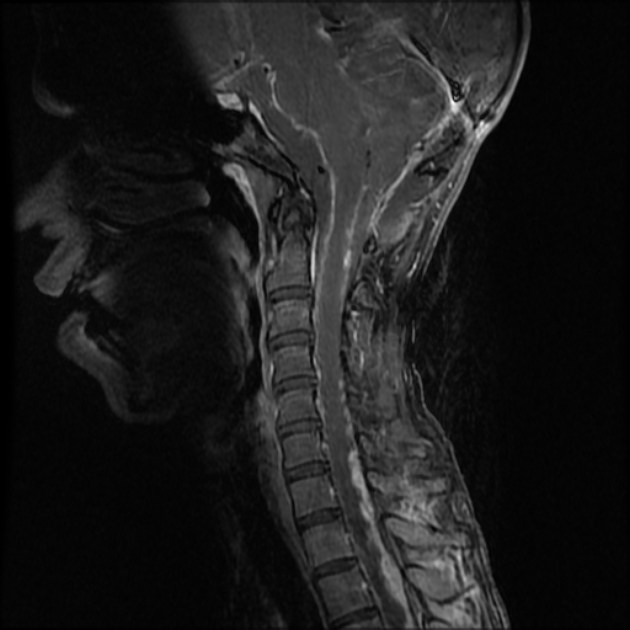
In approximately 40% of patients, there is evidence of CSF seeding at the time of diagnosis 7 and 5% of cases have extra-CNS metastases 21,22.
Pathology
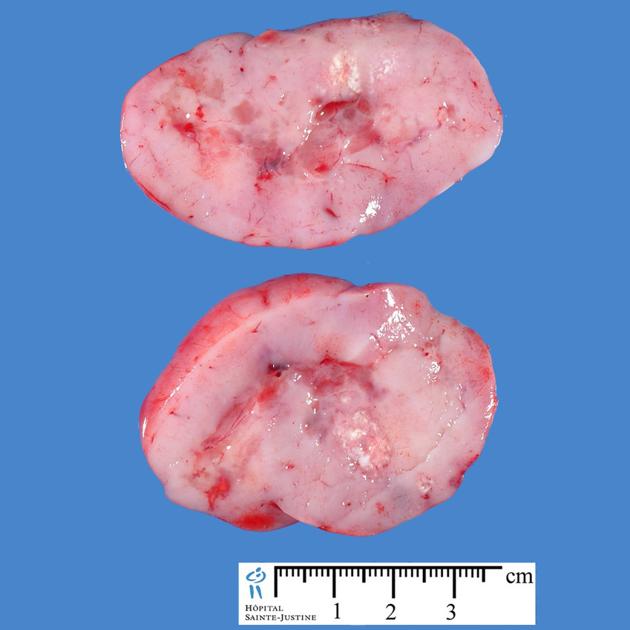
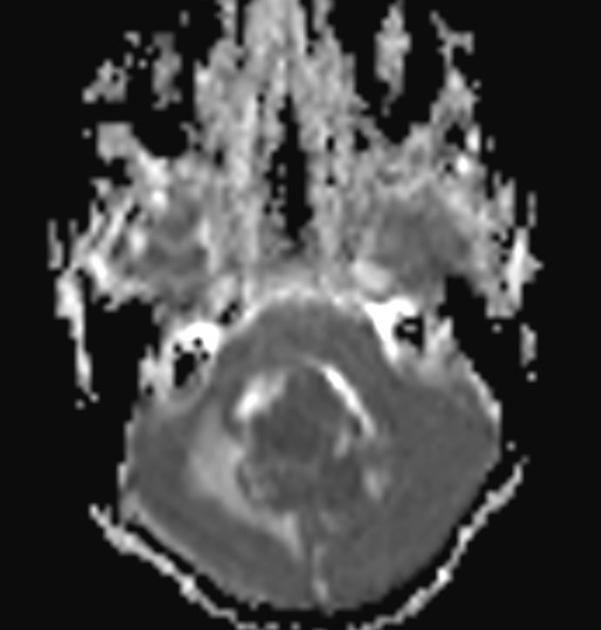
The tumours, in general, tend to be extremely cellular and are an example of a small round blue cell tumour that results in predictable imaging features (diffusion restriction, hyperdense on non-contrast CT, etc.). On light microscopy classic medulloblastoma demonstrates Homer Wright rosettes.
Grading
Although the prognosis of medulloblastomas is variable with some molecular groups (e.g. WNT-activated) having a very high cure rate with appropriate therapy, all medulloblastomas are, at least for now, considered grade 4 tumours 17.
Radiographic features
The radiographic features are strongly influenced by the histological type and molecular subtype of the tumour. Many of the imaging characteristics can, however, be remembered by thinking of medulloblastoma as a small round blue cell tumour.
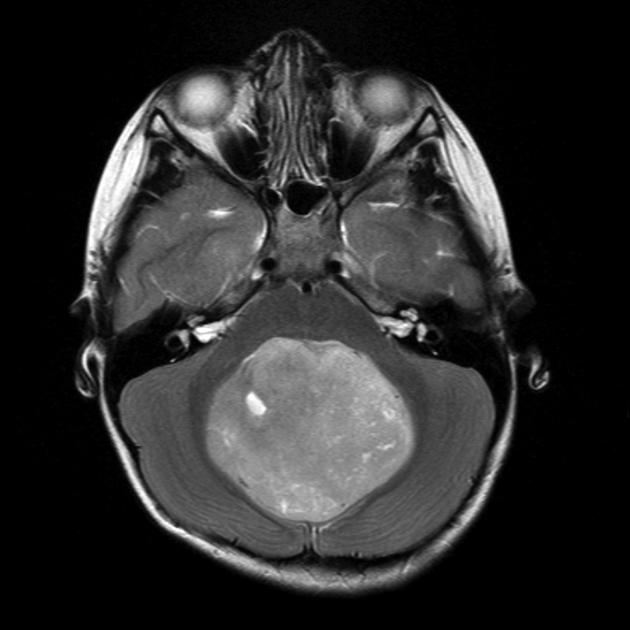
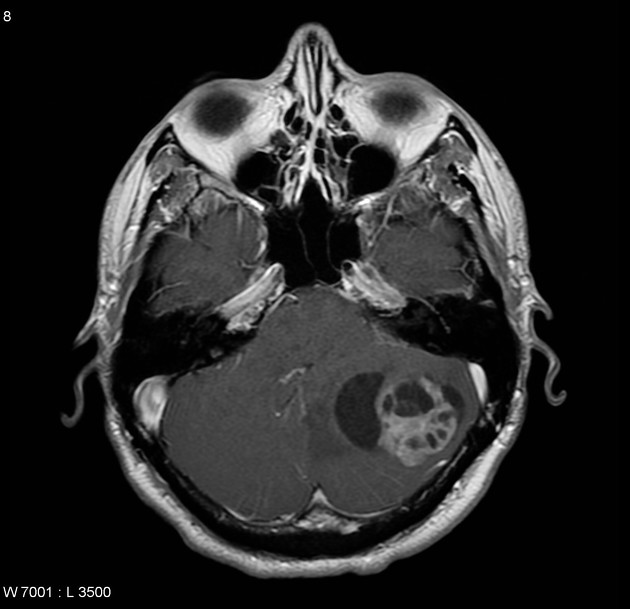
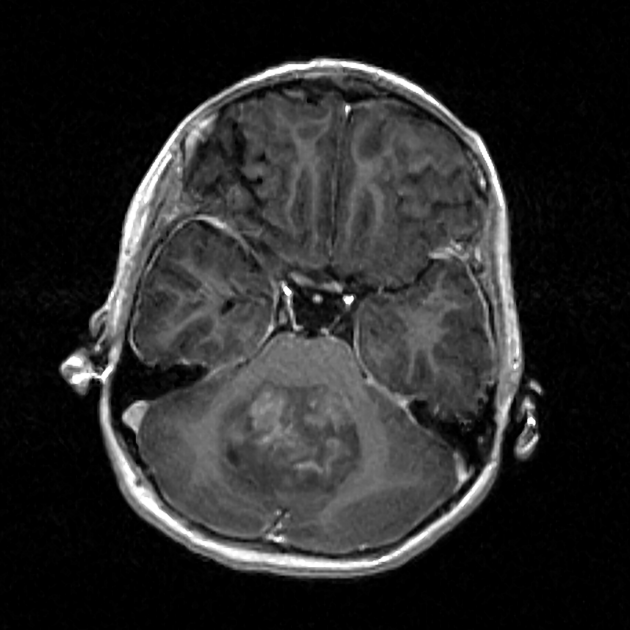
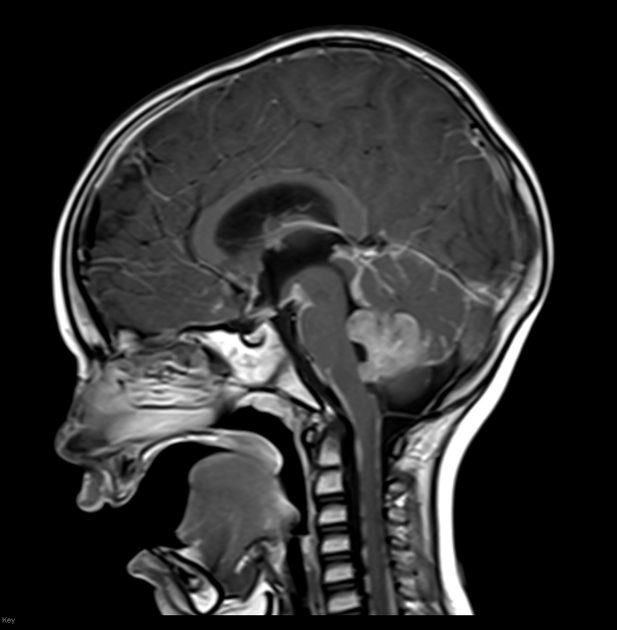
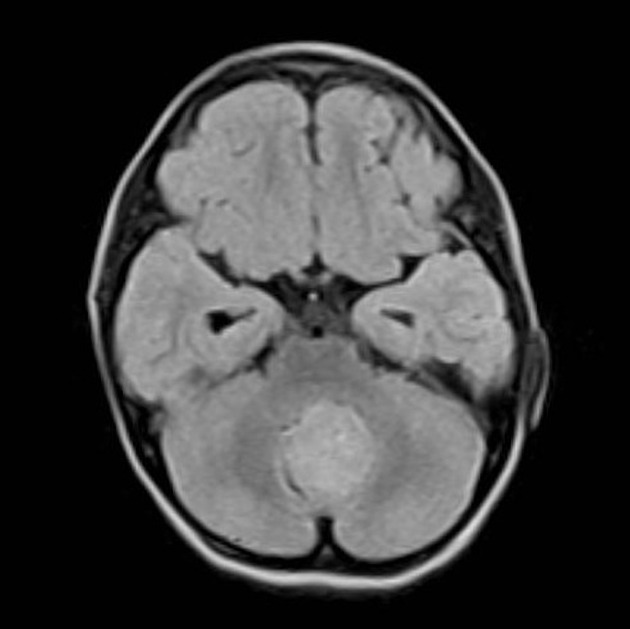
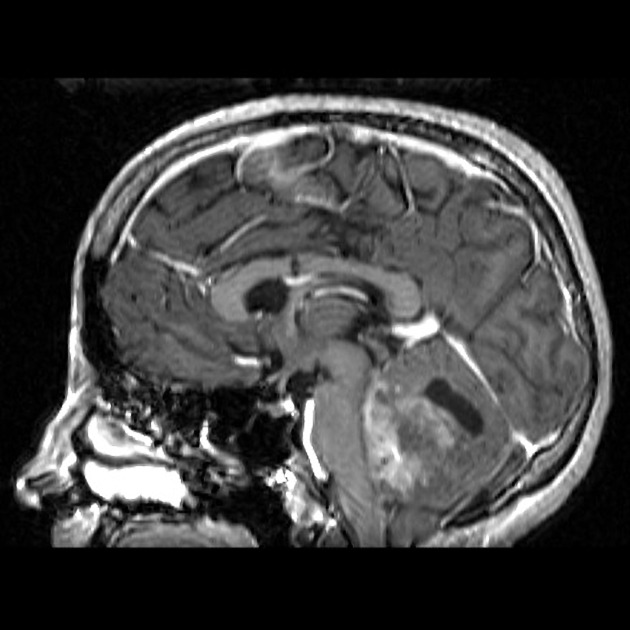
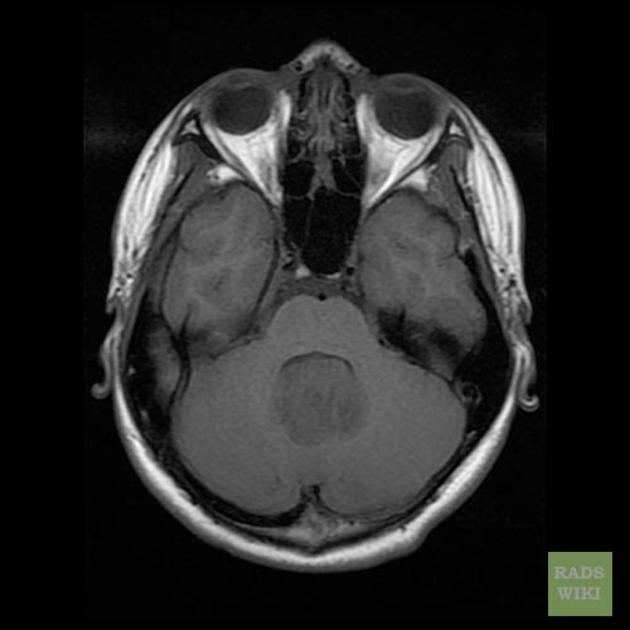
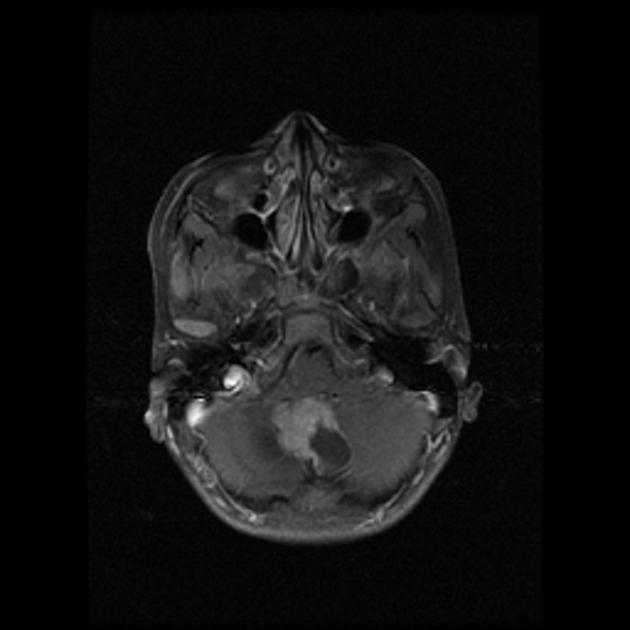
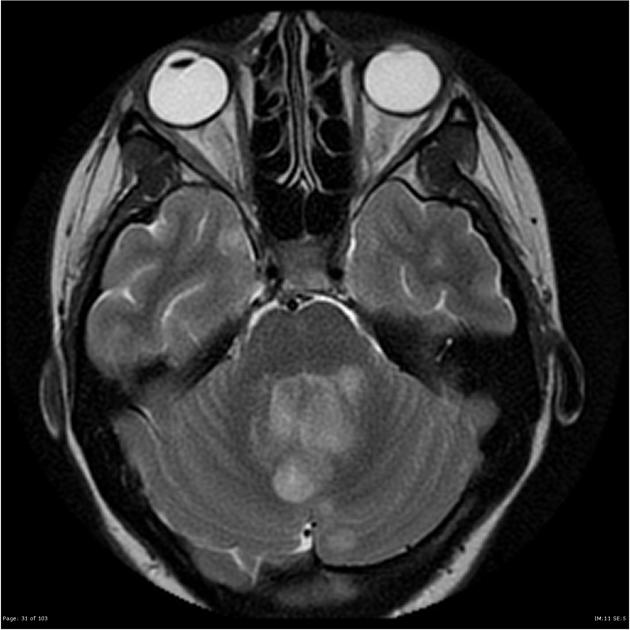
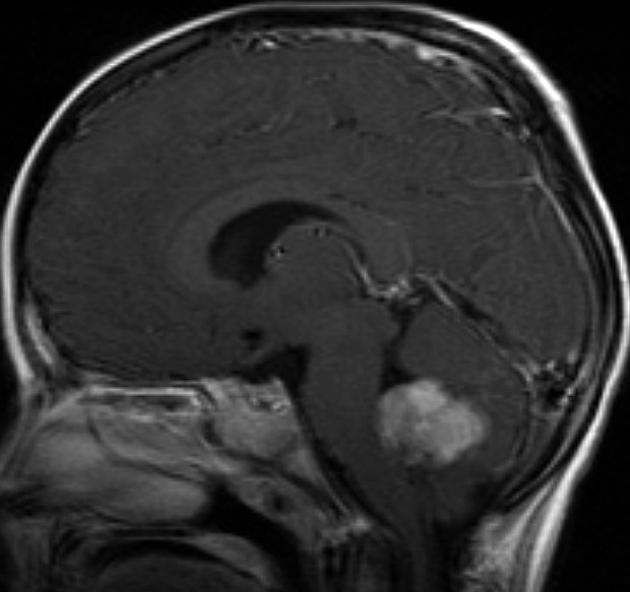
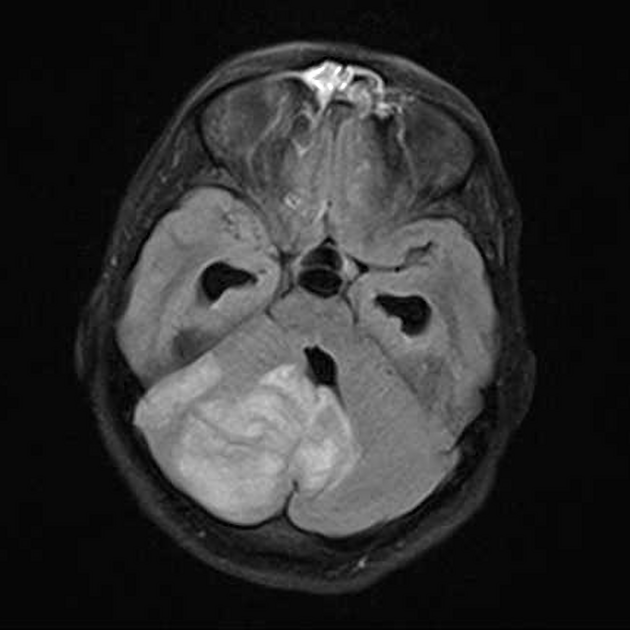
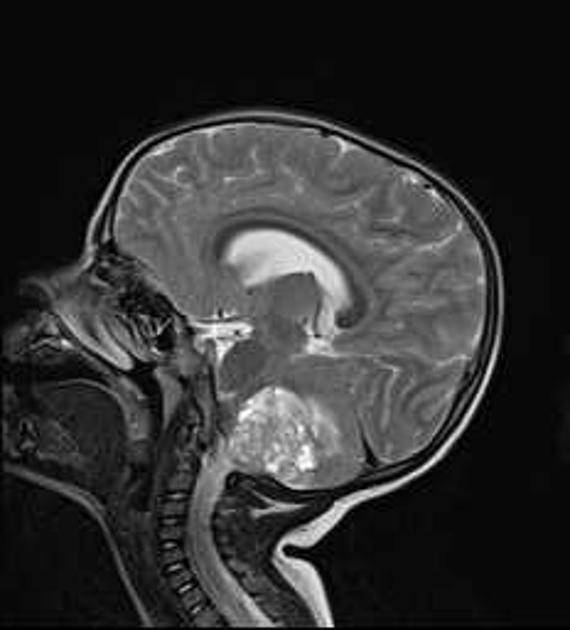
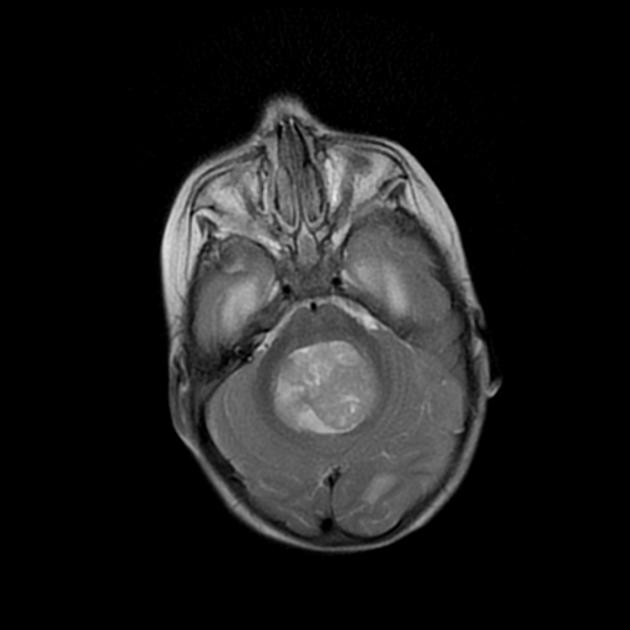
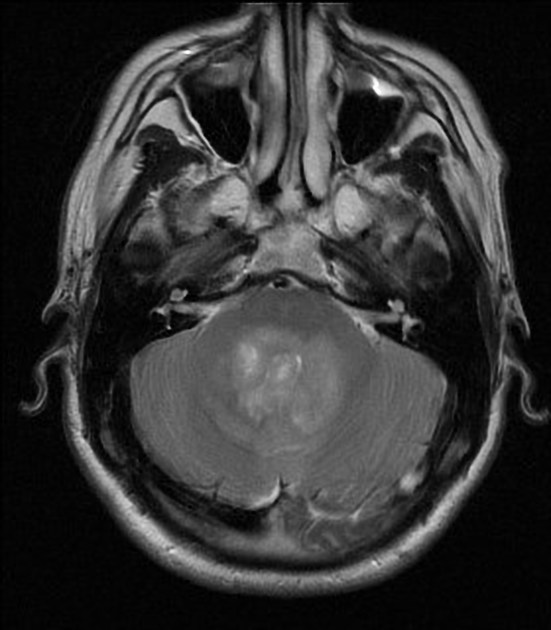
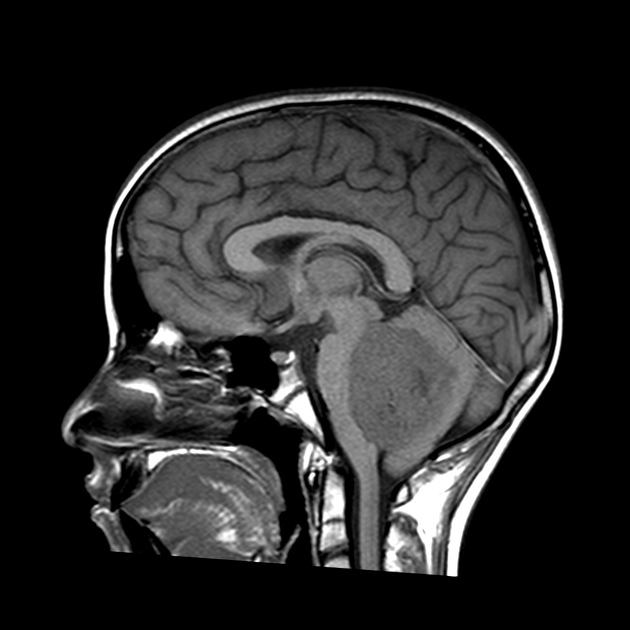
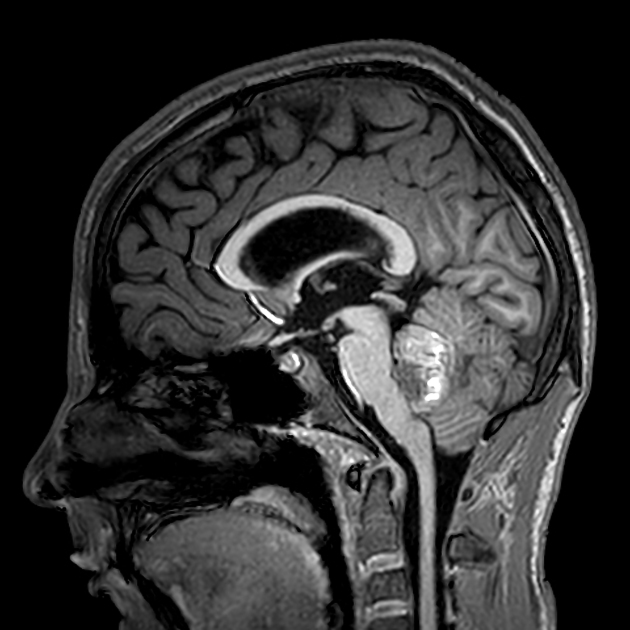
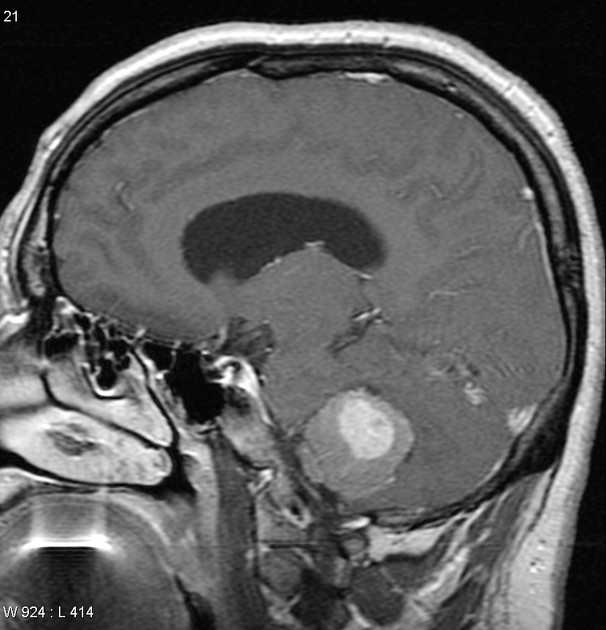
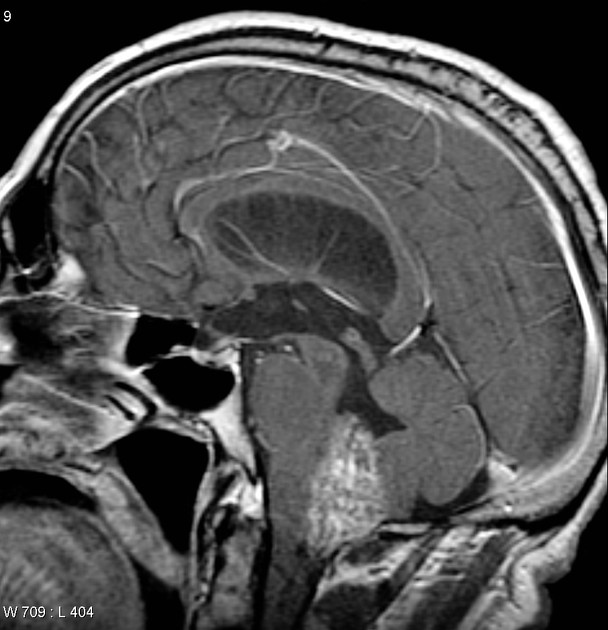
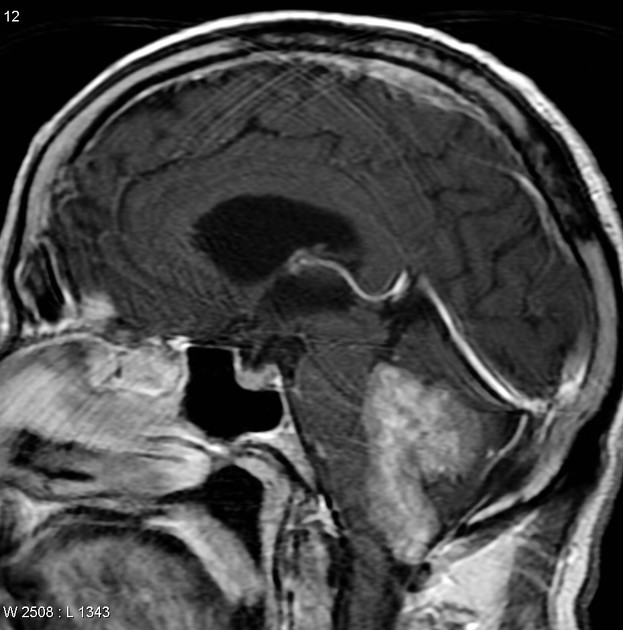
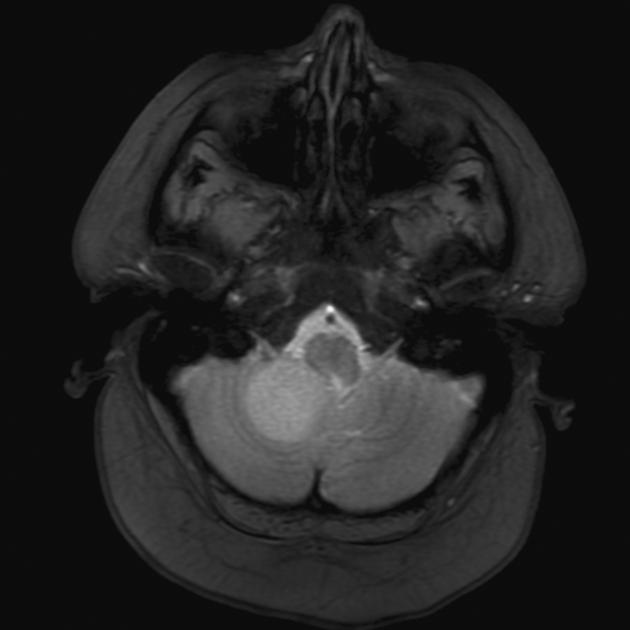
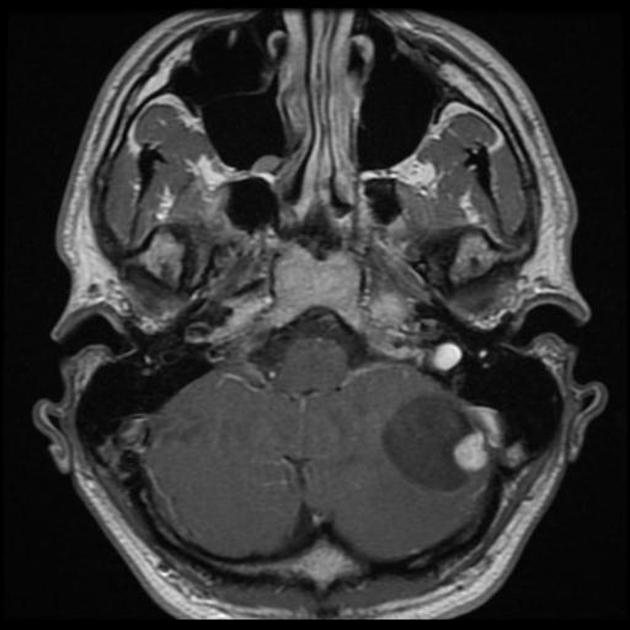
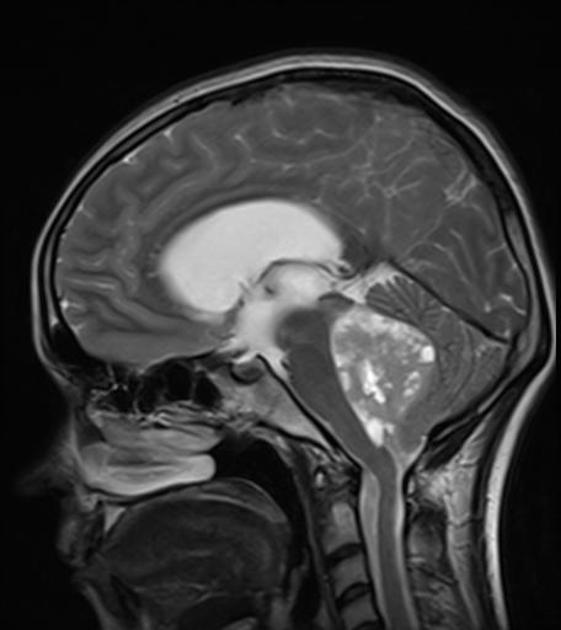
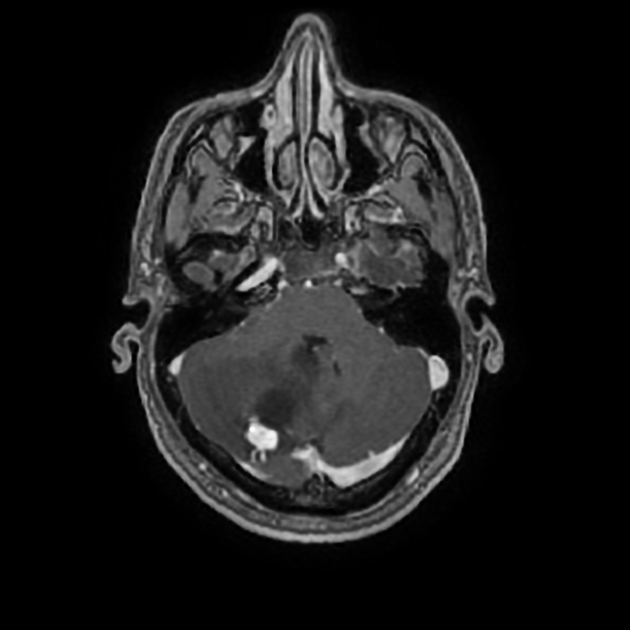
Overall the vast majority (94%) of medulloblastomas arise in the cerebellum and the majority of these, from the vermis (75%). They tend to protrude into the fourth ventricle from its roof (superior medullary velum), and may even grow directly into the brainstem 1,7. This pattern is particularly common in group 3 and group 4 and in some SHH-activated tumours 10.
Other areas are less common and are seen more frequently in older children and adults. In such cases, the tumour is also more likely to be poorly marginated and demonstrate larger cyst formation 7. Adult medulloblastomas are usually located laterally, in the cerebellar hemispheres, with only 28% centred in the vermis; these are most commonly of the SHH-activated tumours 10.
The cerebellar peduncle epicentre is almost exclusively seen in WNT-activated tumours 8-10.
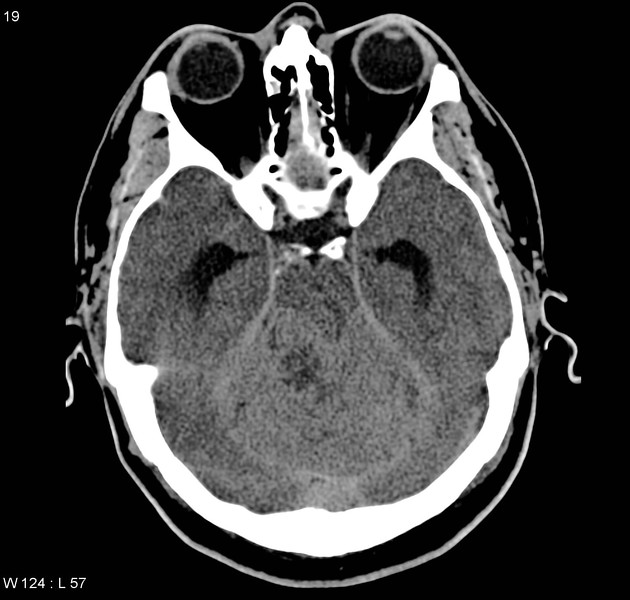
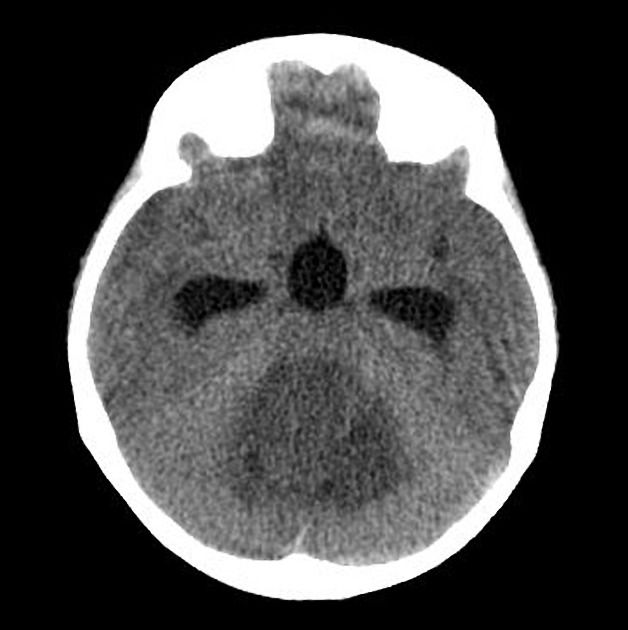
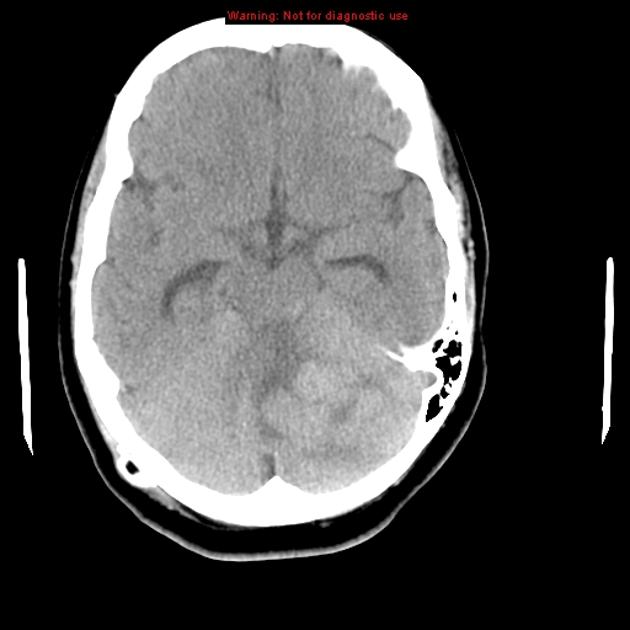
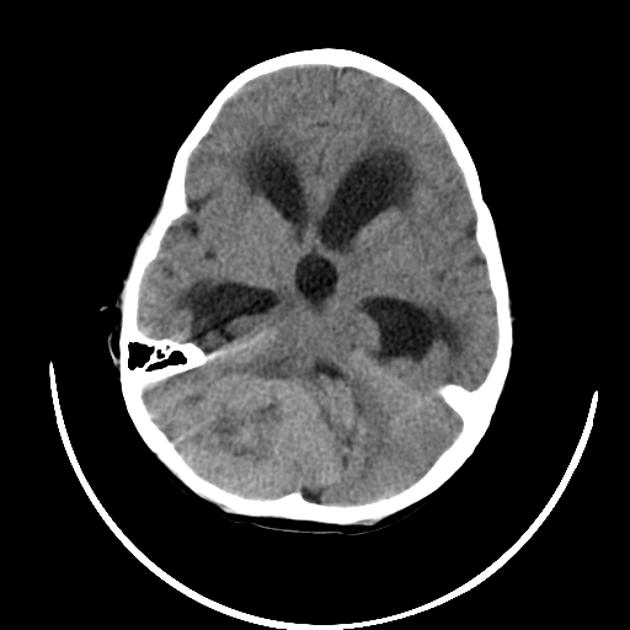
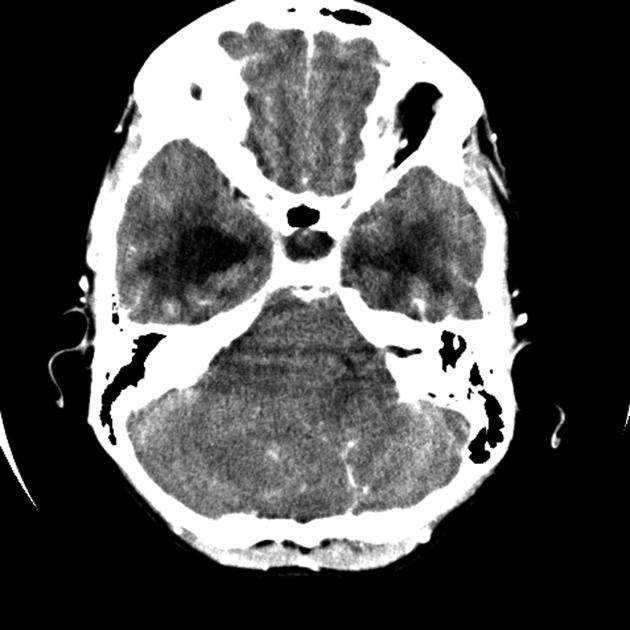
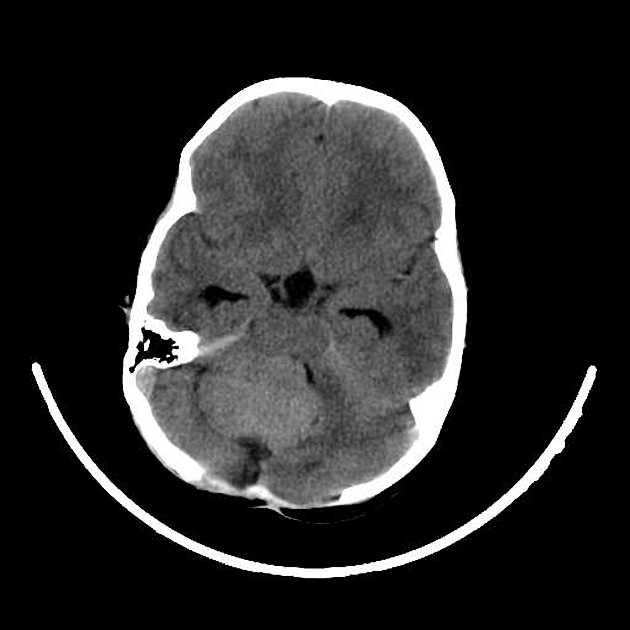
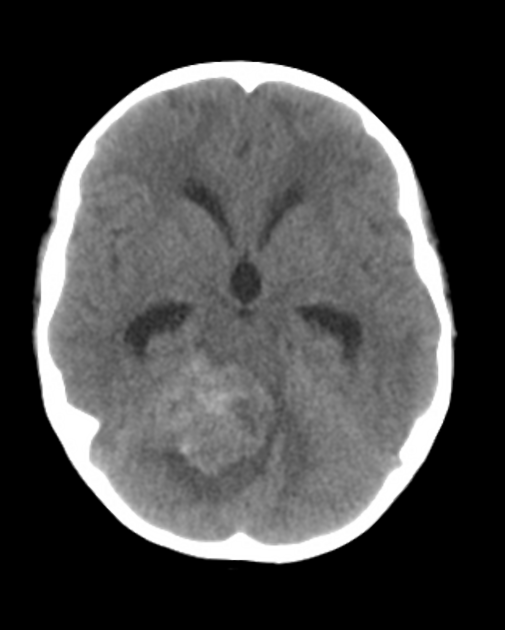
CT
On CT, medulloblastomas often appear as a mass arising from the vermis, resulting in effacement of the fourth ventricle / basal cisterns and obstructive hydrocephalus. They can also occur more laterally in the cerebellum.
They are usually hyperdense (90%) and cysts formation/necrosis is common (40-50%), especially in older patients. Calcification is seen in 10-20% of cases 7.
Enhancement is present in over 90% of cases and is usually prominent 7.
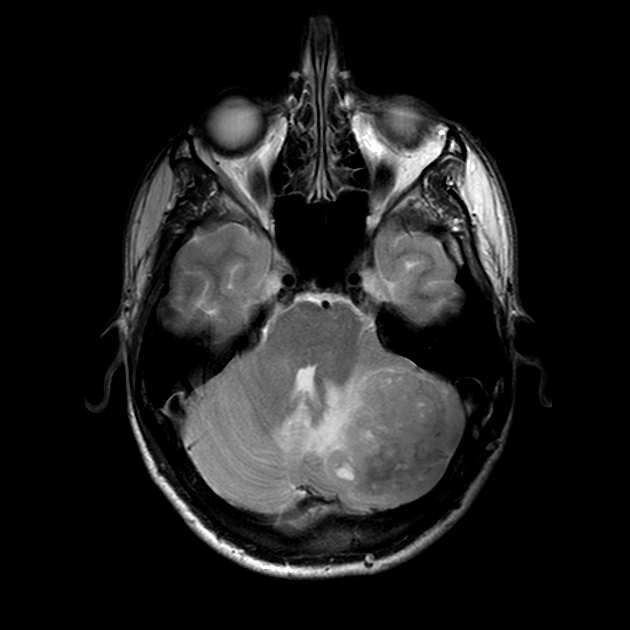
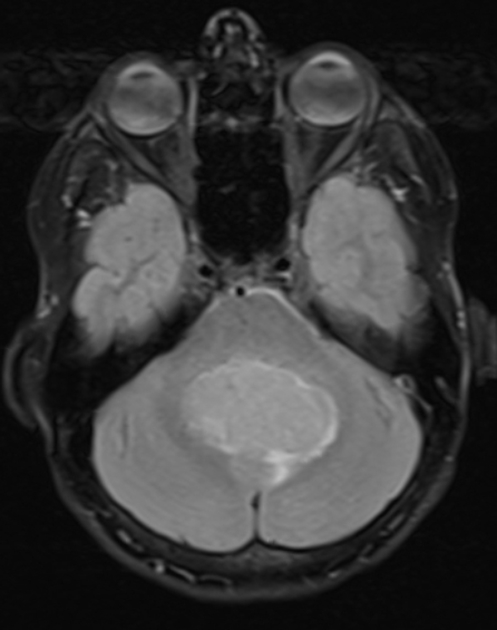
MRI
-
T1
hypointense to grey matter
-
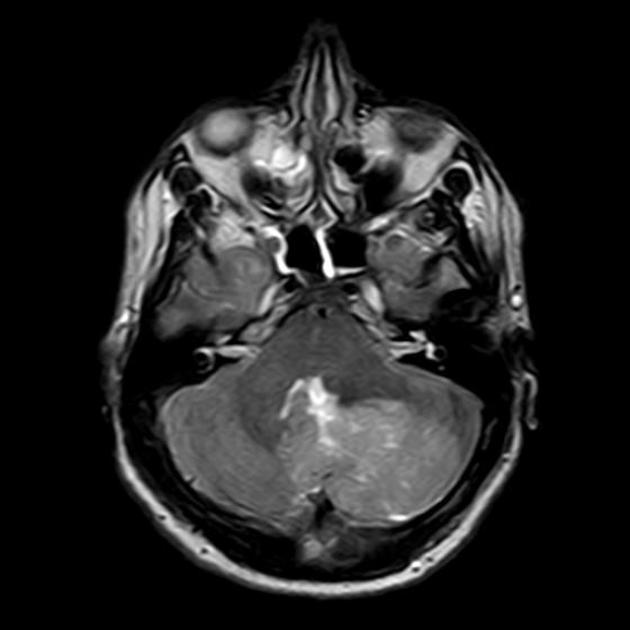
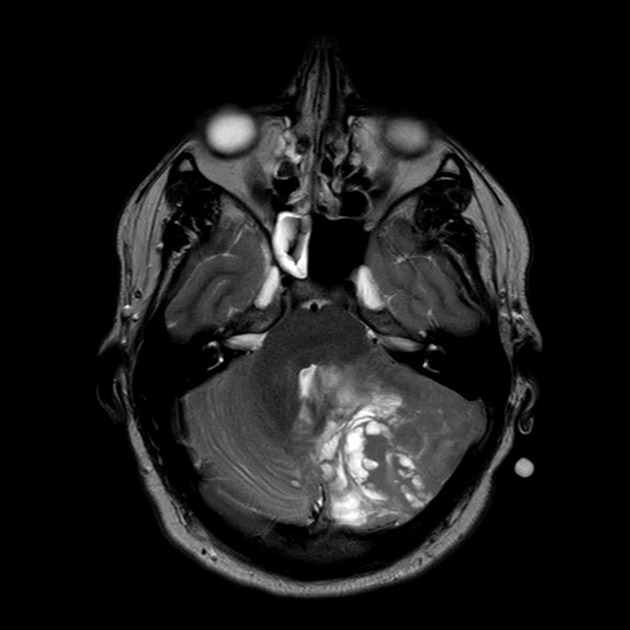
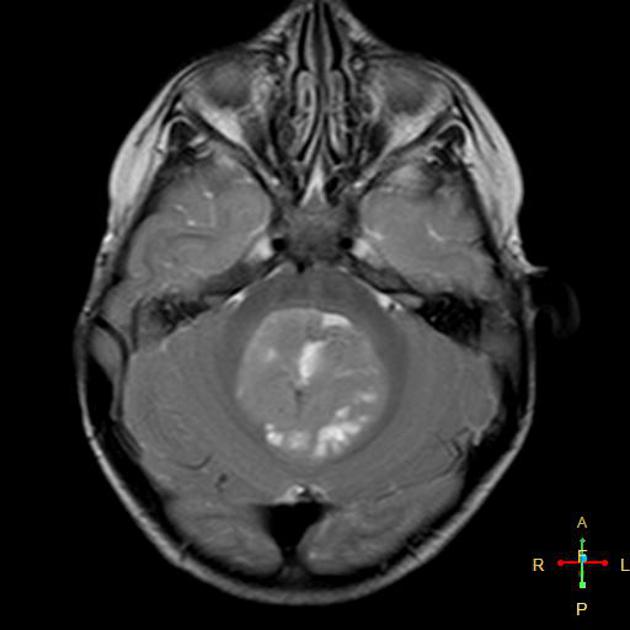
T1 C+ (Gd)
overall 90% enhance, often heterogeneously (tumours in adults tend to have less degree of enhancement compared to the paediatric population24)
WNT-activated tumours tend to vividly enhance 17
group 4 tumours tend to enhance less 10
-
T2/FLAIR
overall are iso to hyperintense to grey matter
heterogeneous due to calcification, necrosis and cyst formation
surrounding oedema is common 10
-
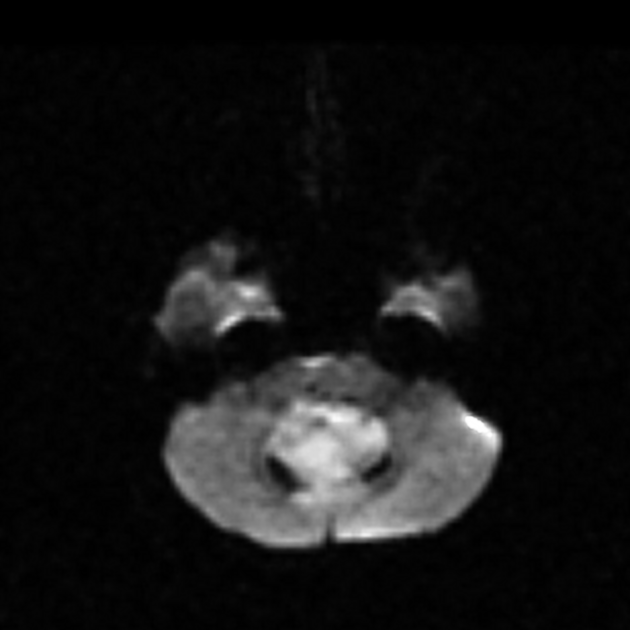
DWI/ADC
high DWI signal ("restricted diffusion") - due to their hypercellularity
low ADC values (lower than normal cerebellum e.g. ~550 x 10-6 mm2/s) 11
-
MR spectroscopy
MRI can delineate the fourth ventricle and subarachnoid space to a much greater degree than CT. Although medulloblastomas project into the fourth ventricle, unlike ependymomas they do not usually extend into the basal cisterns 7.
As CSF seeding is common at presentation, imaging with contrast of the whole neuroaxis is recommended to identify drop metastases and leptomeningeal spread. Although rare, extraneural spread is reported ref.
Predicting molecular subgroup from imaging
So if all this sounds confusing, that's because it is. Molecular subgroups, histology, location, appearance, and demographics all interact, but this notwithstanding, you can make some fairly robust predictions based on imaging when taking all of these together 10,17. Location is the key to this approach.
-
cerebellar peduncle/foramen of Luschka
very likely WNT-activated tumours and therefore best prognosis
-
cerebellar hemisphere
very likely SHH subgroup and therefore intermediate prognosis
likely desmoplastic/nodular/medulloblastoma with extensive nodularity (MBEN)
-
midline
may be group 3, group 4 or SHH
typically infants with a tumour with ill-defined margins but prominent enhancement: likely group 3 (or SHH) and therefore worst prognosis
typically children with a tumour with well-defined margins but mild or no enhancement: likely group 4 and therefore slightly better prognosis
adults with variably defined and variably enhancing tumours: most likely SHH; haemorrhage raises the probability of group 4 13
MR spectroscopy may also be distinctive 5,14:
-
group 3 or 4
taurine peak
high creatine
-
SHH
little or no taurine
low creatine
Treatment and prognosis
Treatment typically consists of surgical resection, radiation therapy, and chemotherapy. In general, the tumours are quite radiosensitive. A VP shunt is required in approximately 35% of children who undergo resection.21
Cerebellar mutism syndrome may occur after surgical resection of medulloblastoma 16.
Prognosis is most strongly influenced by molecular subtype 7-9:
WNT: very good
SHH: infants good, others intermediate
group 3: poor
group 4: intermediate
Traditionally, and still relevant in addition to molecular characteristics, prognosis if considered poor if young age at diagnosis (<3 years of age), incomplete surgical resection (>1.5 cm3 residual), and presence of CSF metastases at the time of diagnosis; common in infants and children (~25%) and uncommon in adults (~2%) 1,12.
Histological features also have an effect on prognosis with desmoplastic and nodular histology having a better outlook than large cell or anaplastic histologic features 12.
Expression of the c-erbB-2 (HER2/neu) oncogene is useful in the staging of medulloblastomas. The increased c-erbB-2 expression reflects an increase in the proliferative activity of a tumour (widely used in breast cancer staging).
no CSF metastases, complete surgical resection and negative c-erbB-2 expression: 5-year-survival 100%
no CSF metastases, complete surgical resection and positive c-erbB-2 expression: 5-year-survival 54%
CSF metastases and/or incomplete surgical resection: 5-year-survival 20%
History and etymology
In 1925, Bailey and Cushing described the first cases of medulloblastomas 1.
Differential diagnosis
In the paediatric population, consider the following alternative diagnoses:
-
usually arises from the floor of the 4th ventricle
typically squeezes out the foramen of Luschka
does not usually cause as much diffusion restriction
-
atypical teratoid/rhabdoid tumour
very young children
aggressive
-
large cystic component
brightly enhancing mural nodule
brainstem glioma (exophytic)
choroid plexus papilloma (CPP): more common in lateral ventricles in children
In the adult population, consider the following:
See also:







 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.