Oesophageal carcinoma is globally the 7th most common cancer and 6th most common cause of cancer-related death as per NCCN version 3.2023. It tends to present with increasing dysphagia, initially to solids and progressing to liquids as the tumour increases in size, obstructing the lumen of the oesophagus.
On this page:
Epidemiology
Oesophageal cancer is responsible for <1% of all cancers and 4-10% of all gastrointestinal malignancies. There is recognised male preponderance with the squamous cell subtype, M:F 4:1.
The incidence of the subtypes has regional variation. The squamous cell subtype has the greatest worldwide incidence (~90%), but the adenocarcinoma subtype is more common in many parts of North America and Europe. In addition, there are certain regions where individuals are at particularly high risk of developing oesophageal cancer, e.g. Iran, Malawi, Zimbabwe, Mongolia, Italy, and China.
Predisposing factors include 8:
alcohol and smoking: for squamous cell carcinoma and adenocarcinoma
Barrett oesophagus: for adenocarcinoma
caustic stricture/lye stricture
obesity: for adenocarcinoma
history of oral or pharyngeal cancer
human papillomavirus (HPV)
tylosis (Howel–Evans syndrome): a rare autosomal dominant disease with hyperkeratosis of the palms and soles with a high incidence of oesophageal squamous cell carcinoma
Bloom syndrome: rare autosomal recessive disorder consisting of haematological malignancies Wilms tumour and solid tumours, including that of the oesophagus
Fanconi anaemia: a rare autosomal recessive disorder characterised by haematological malignancies, pancytopenia, congenital malformations and solid tumours (such as that of the oesophagus)
Clinical presentation
Patients present with progressive dysphagia, weight loss, chronic worsening gastro-oesophageal reflux and hoarseness, cough, vocal cord paralysis, or other signs and symptoms of mediastinal invasion.
Pathology
Histological types
squamous cell carcinoma of the oesophagus: 81-95% (worldwide)
-
adenocarcinoma of the oesophagus: 4-19% (worldwide)
arising from mucosal/submucosal glands, heterotopic gastric mucosa, or columnar-lined epithelium
>90% related to Barrett oesophagus
tend to occur at the gastro-oesophageal junction
-
other types
* in the western world, adenocarcinoma is as common or even slightly more common than squamous cell carcinoma
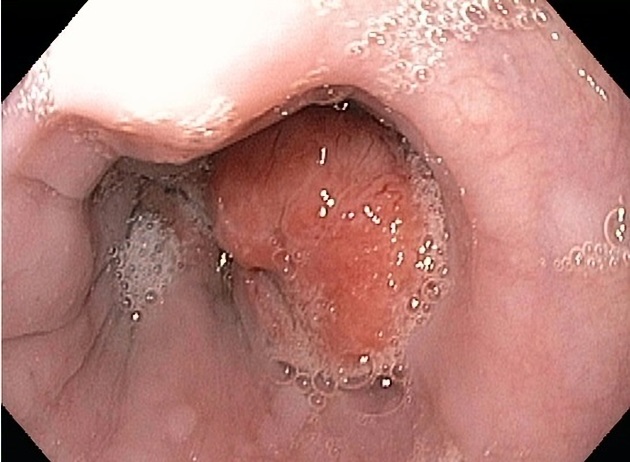
Macroscopic appearance
-
polypoid/fungating (most common)
sessile/pedunculated tumour
lobulated surface protruding
irregular, polycyclic, overhanging, step-like "apple core" lesion
ulcerating: large ulcer niche within a bulging mass
infiltrating: gradual narrowing with a smooth transition
superficial spreading carcinoma
Staging
See the separate articles by histology:
oesophageal and oesophagogastric junction adenocarcinoma (staging)
oesophageal and oesophagogastric junction squamous cell carcinoma (staging)
oesophageal and oesophagogastric junction neuroendocrine tumour (staging)
Metastases
-
lymphatic
anterior jugular chain and supraclavicular nodes (primary in upper 1/3)
para-oesophageal and subdiaphragmatic nodes (primary in middle 1/3)
mediastinal and paracardiac and coeliac trunk nodes (primary in lower 1/3)
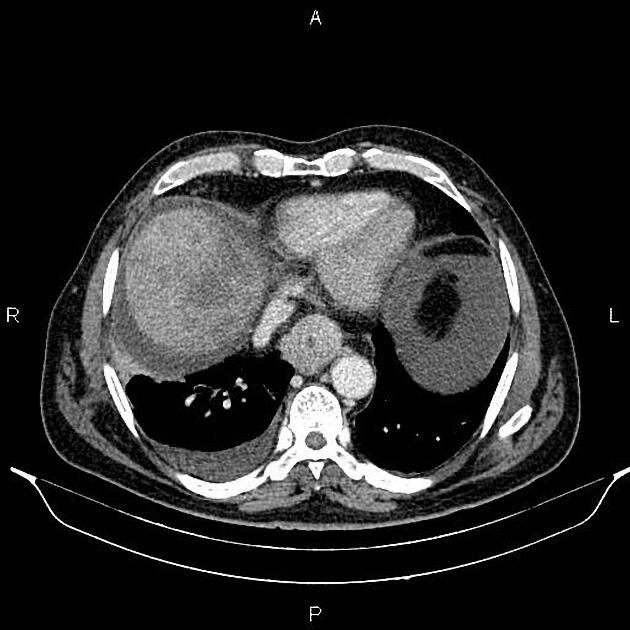
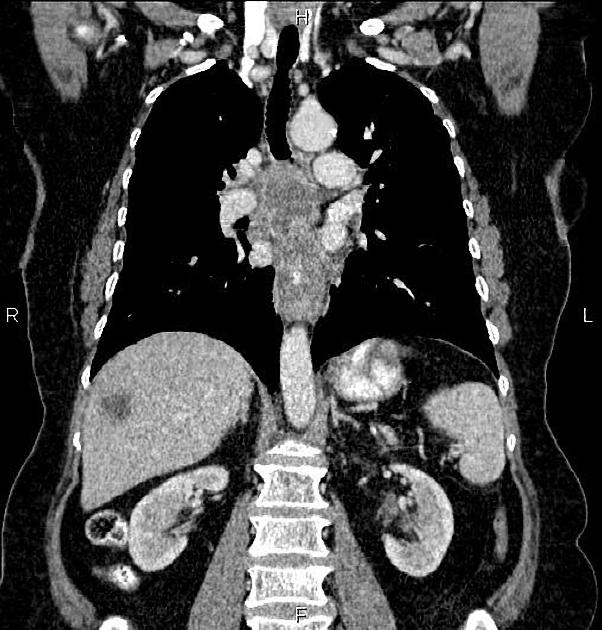
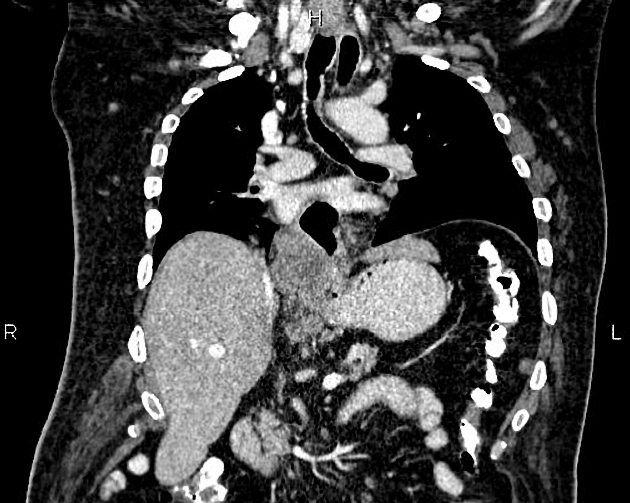
haematogenous: lung, liver, adrenal glands
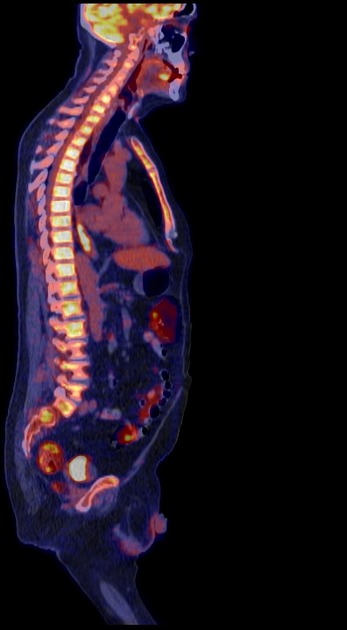
Radiographic features
A combination of CT scan, transoesophageal ultrasound, and PET-CT is used to stage the disease. CT is the best initial modality for detecting distant metastasis, gross direct invasion, and enlarged lymph nodes. Ultrasound is the most sensitive modality for assessment of the depth of invasion and regional enlarged lymph nodes. PET can be useful for restaging after the initial neoadjuvant therapy 7.
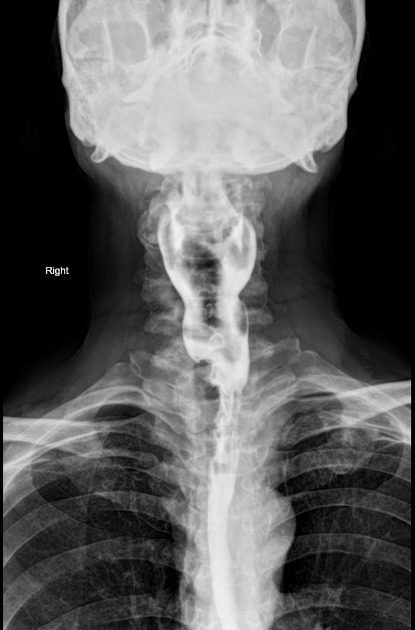
Plain radiograph
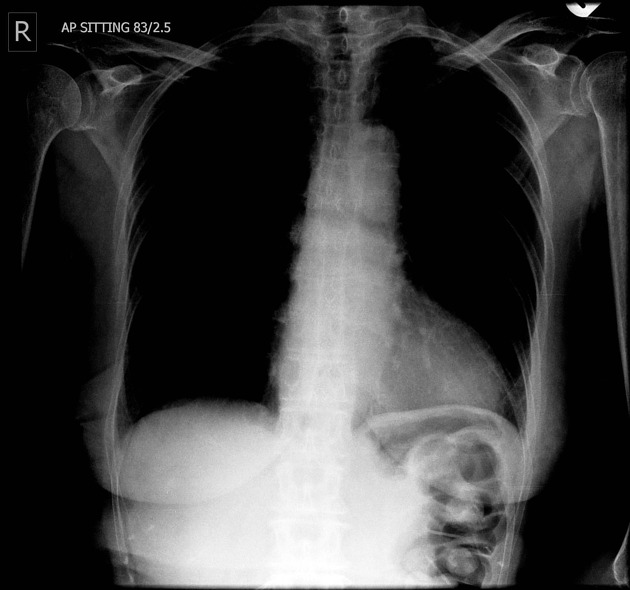
Chest radiograph
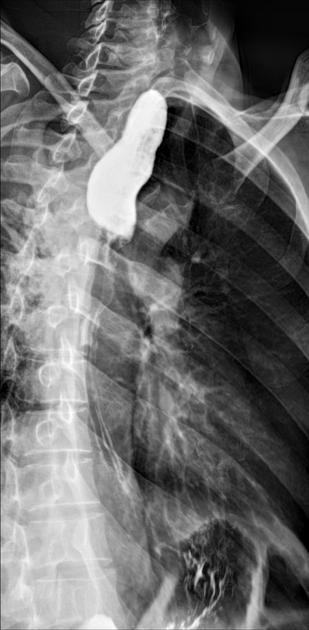
Many indirect signs can be sought on a chest radiograph, and these include:
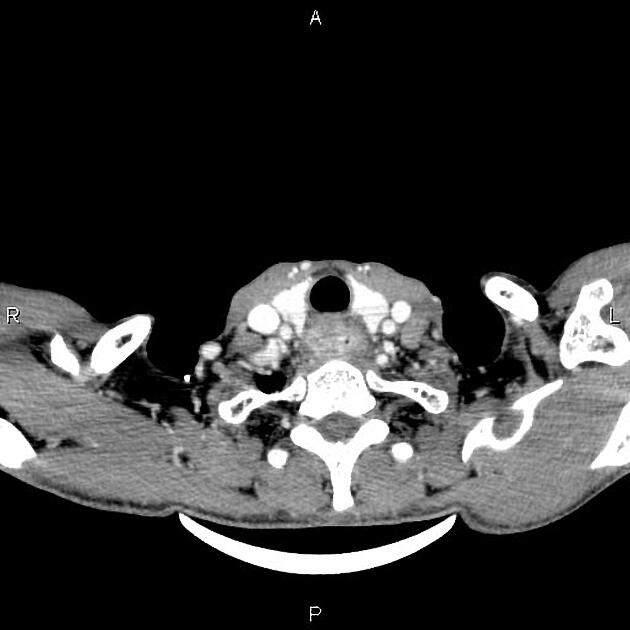
widened azygos-oesophageal recess with convexity toward the right lung (in 30% of distal and mid-oesophageal cancers)
thickening of posterior tracheal stripe and right paratracheal stripe >4 mm (if tumour located in the upper third of oesophagus)
tracheal deviation or posterior tracheal indentation/mass
retrocardiac or posterior mediastinal mass
oesophageal gas-fluid level
lobulated mass extending into a gastric bubble (Kirklin sign)
repeated aspiration pneumonia (with tracheo-oesophageal fistula)
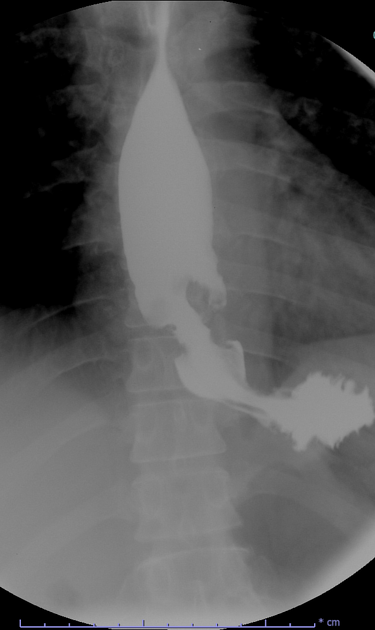
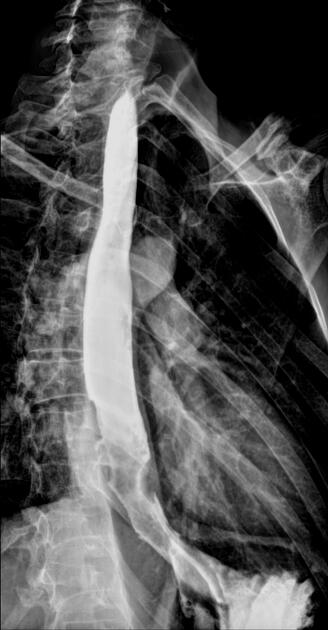
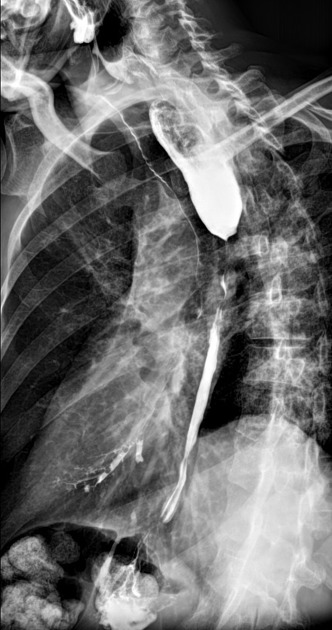
Fluoroscopy
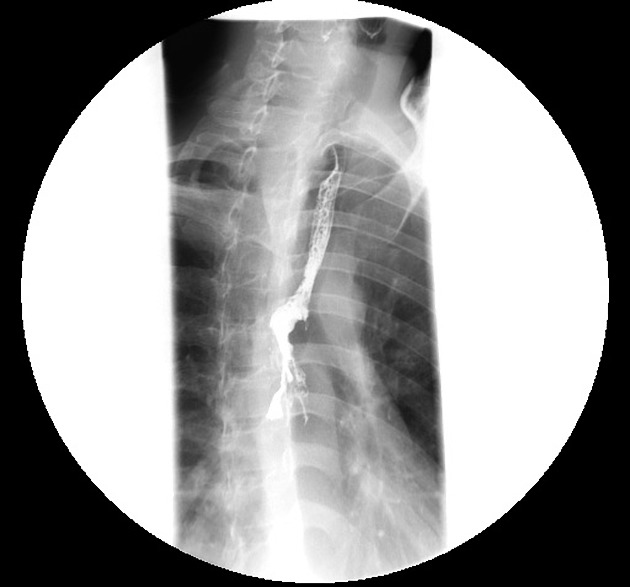
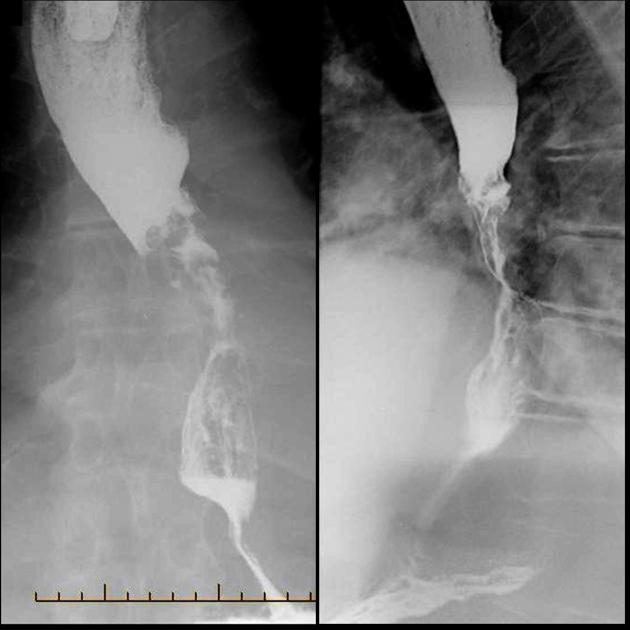
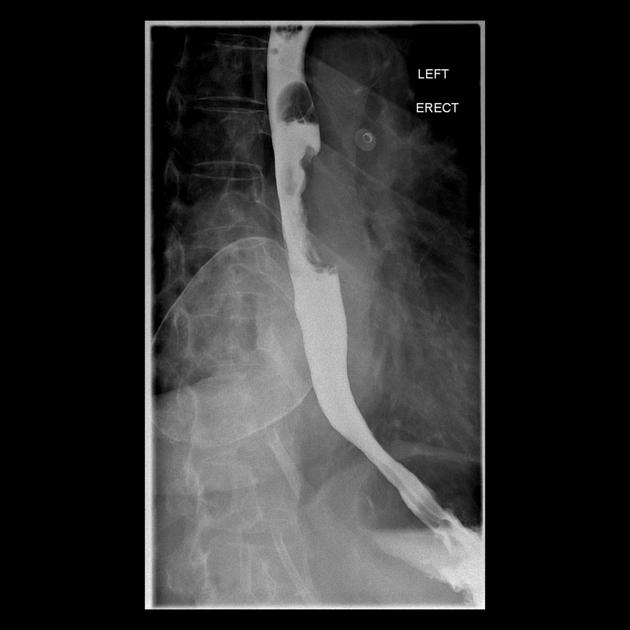
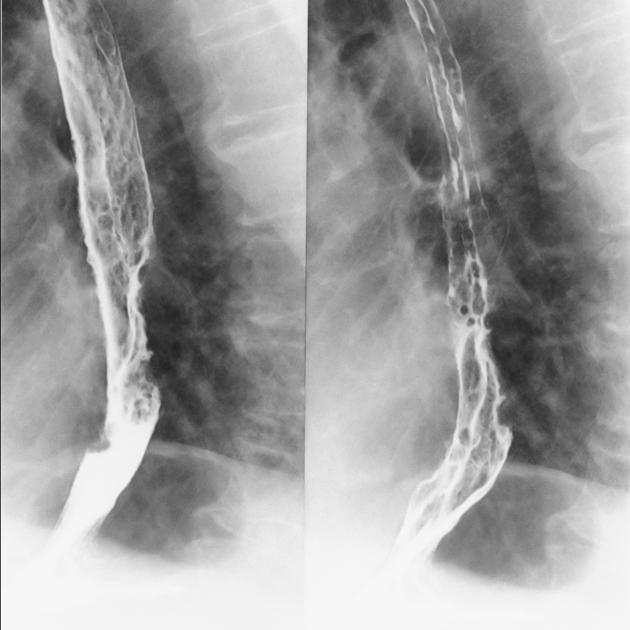
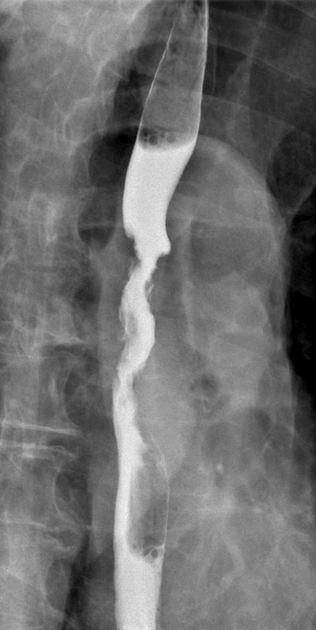
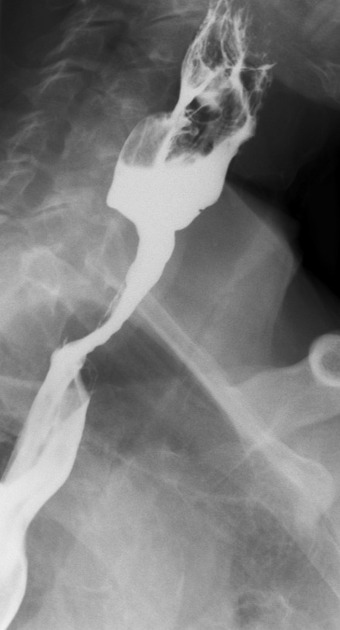
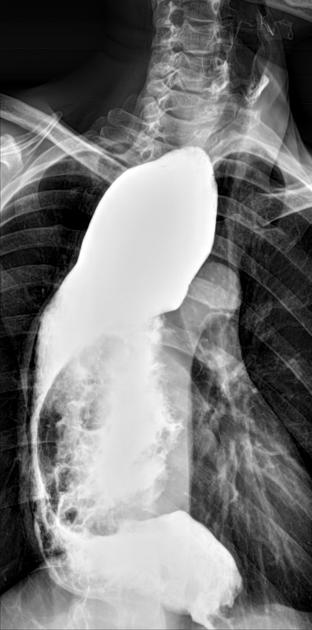
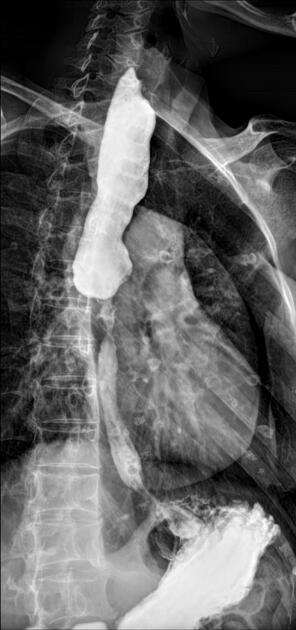
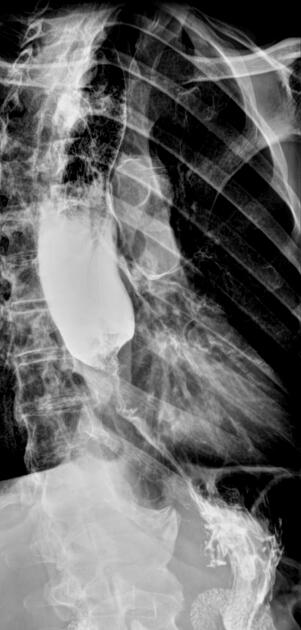
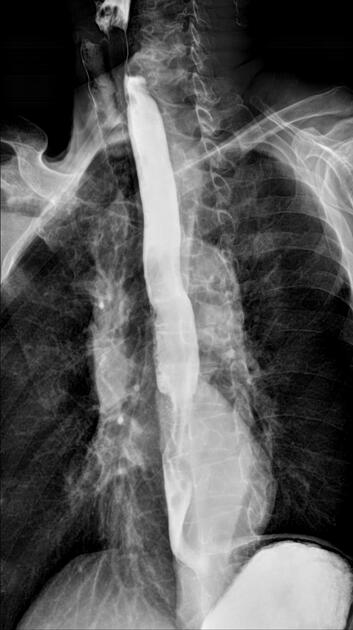
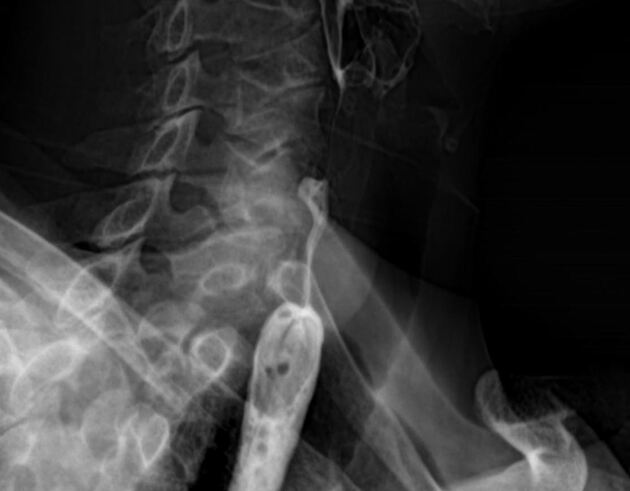
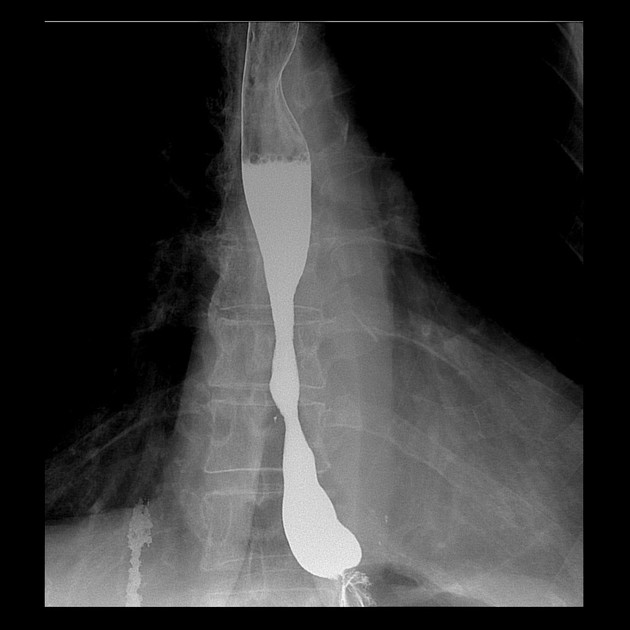
Contrast swallow
irregular stricture
prestricture dilatation with 'hold up'
shouldering of the stricture
US
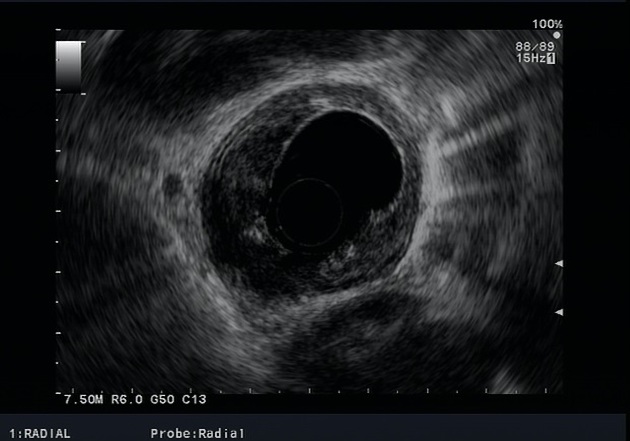
Endoscopic ultrasound
The most accurate imaging modality for the T staging of oesophageal cancer. It defines the layers of the oesophageal wall hence can differentiate T1, T2, and T3 tumours.
The oesophagus consists of five layers:
first hyperechoic layer represents the interface between the balloon and the superficial mucosa
second hypoechoic layer represents the lamina propria and muscularis mucosae
a third hyperechoic layer represents the submucosa
fourth hypoechoic layer represents the muscularis propria
fifth layer represents the interface between the adventitia and surrounding tissues
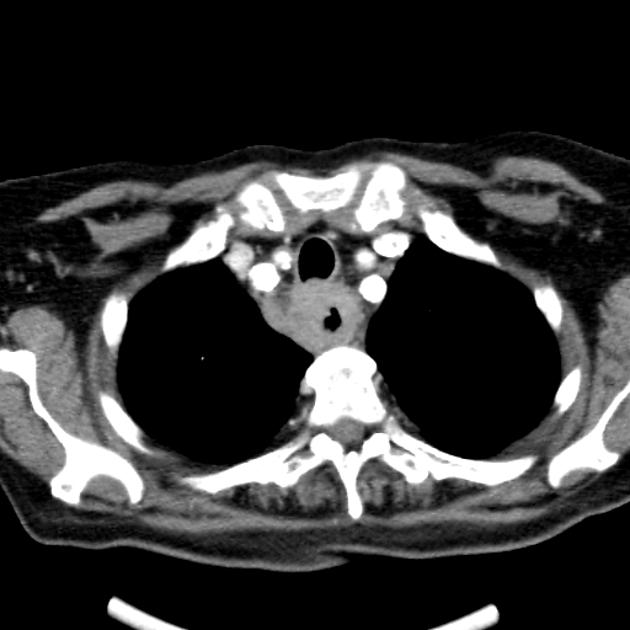
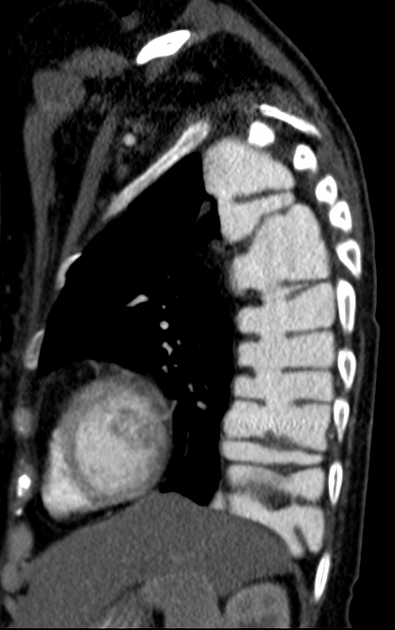
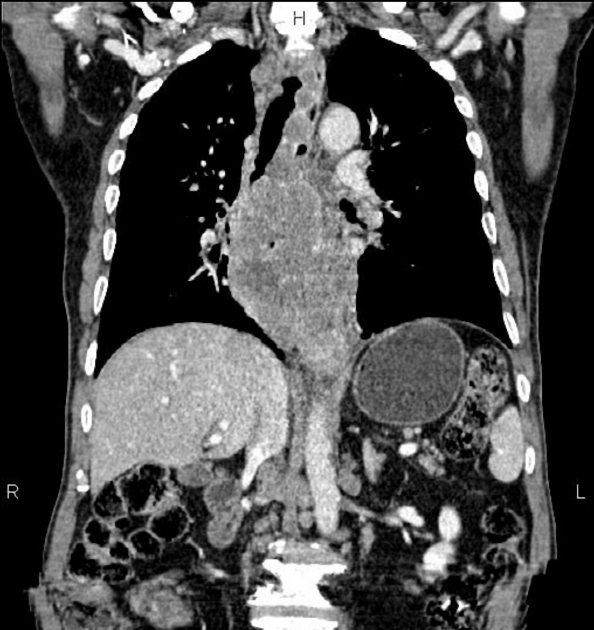
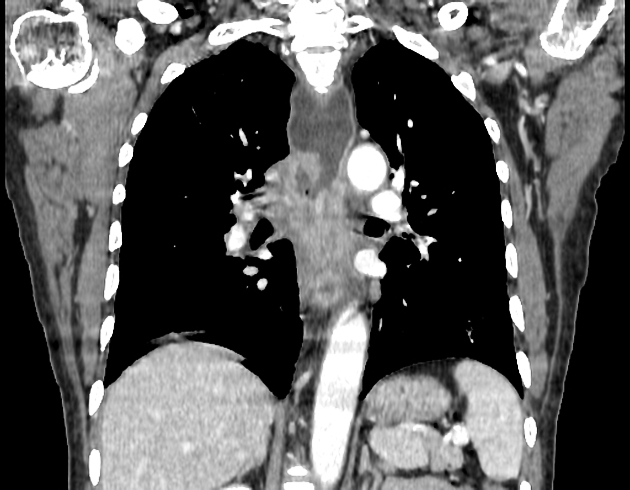
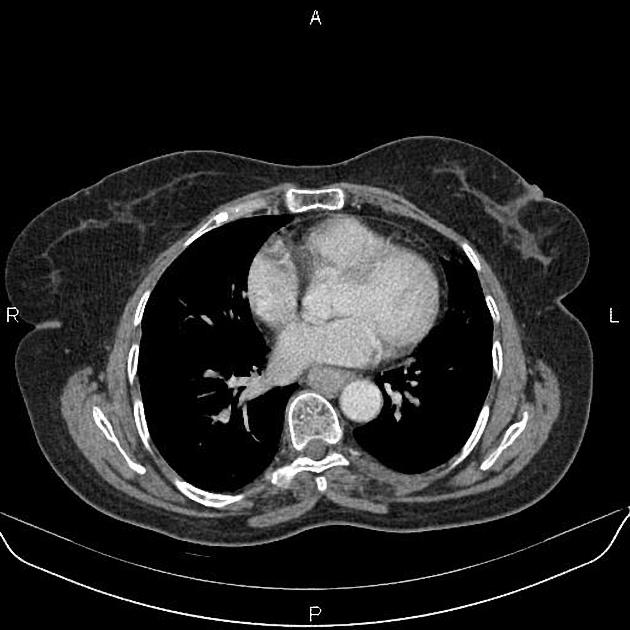
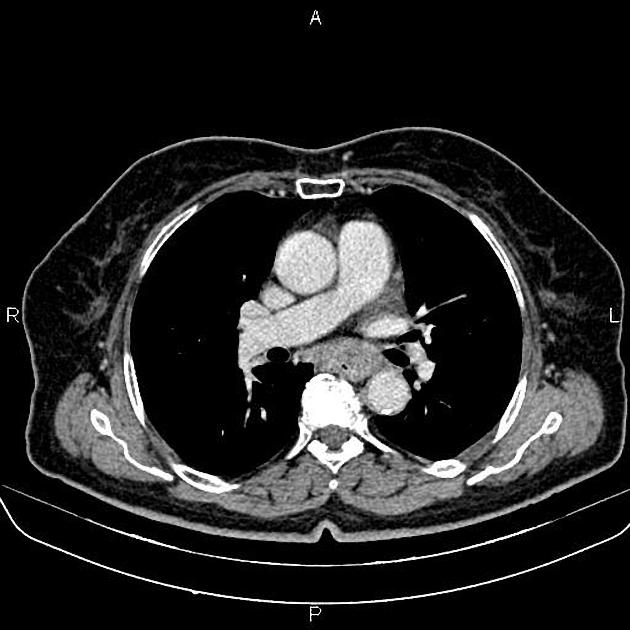
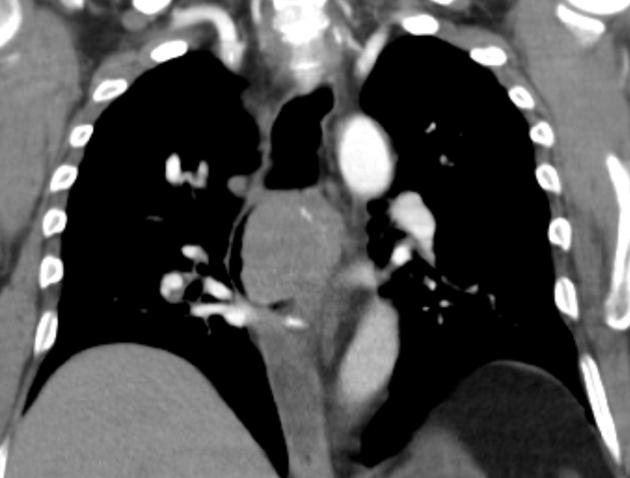
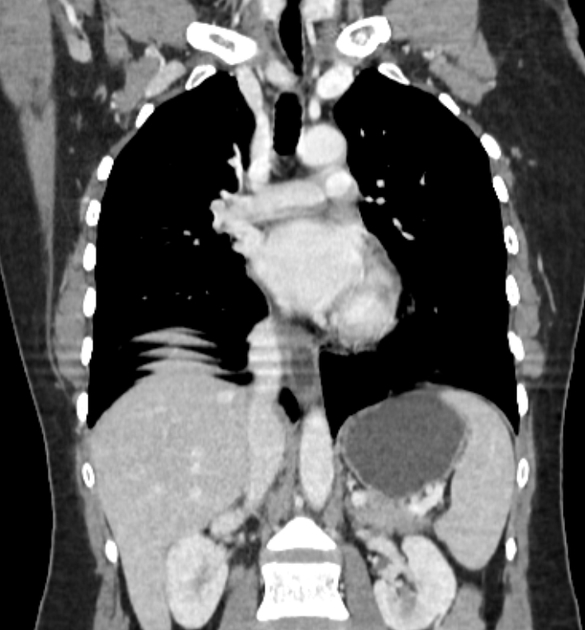
CT
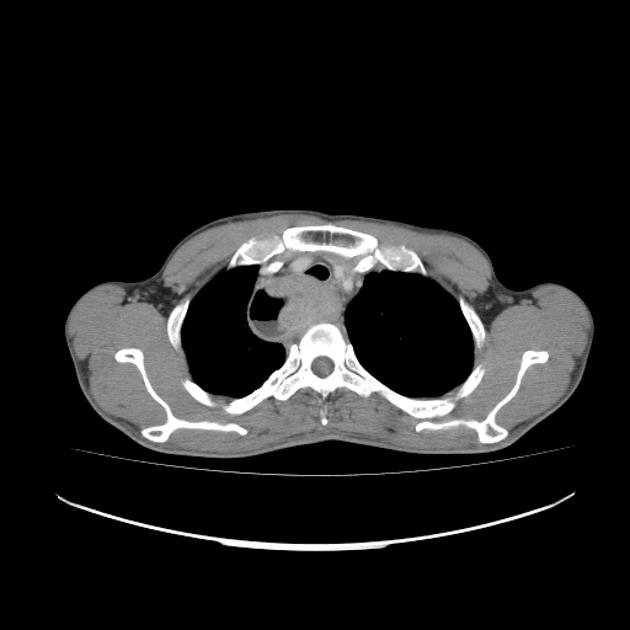
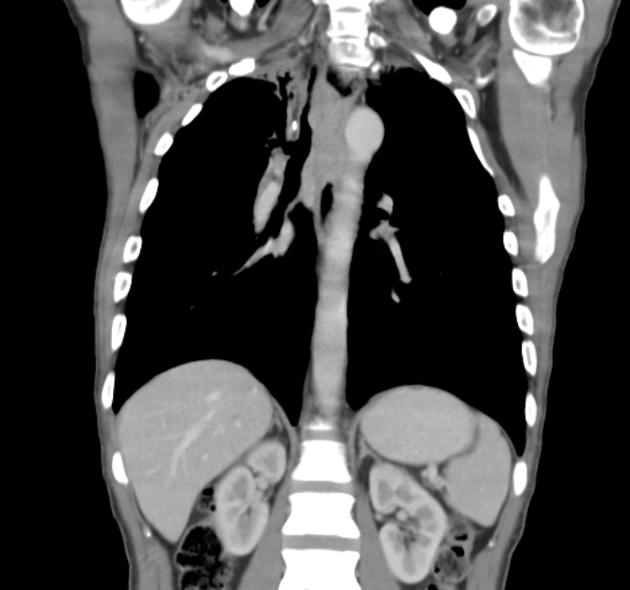
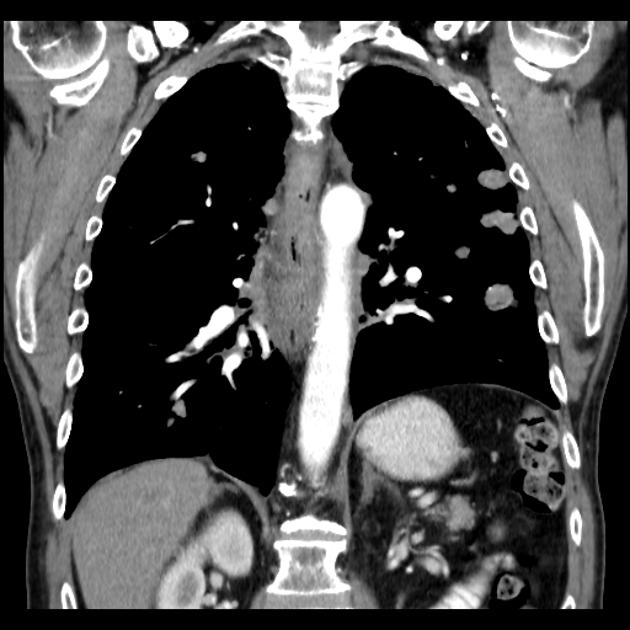
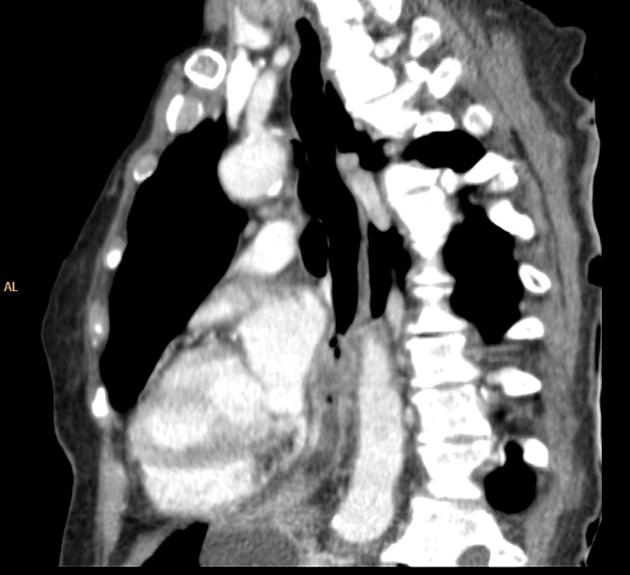
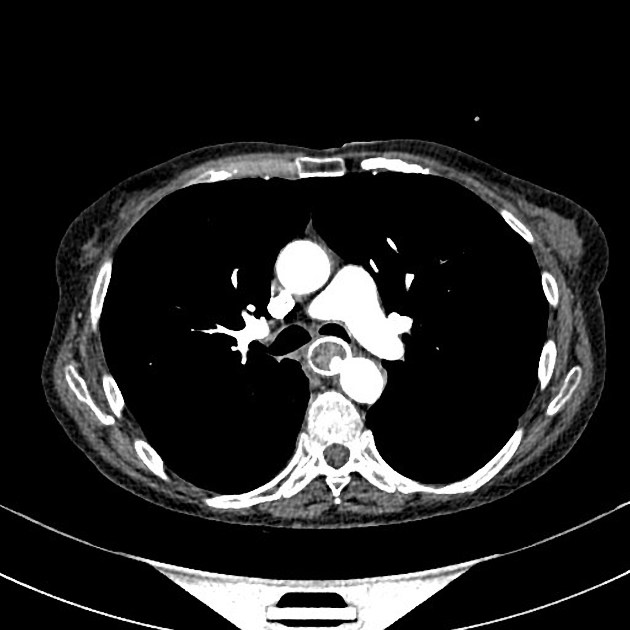
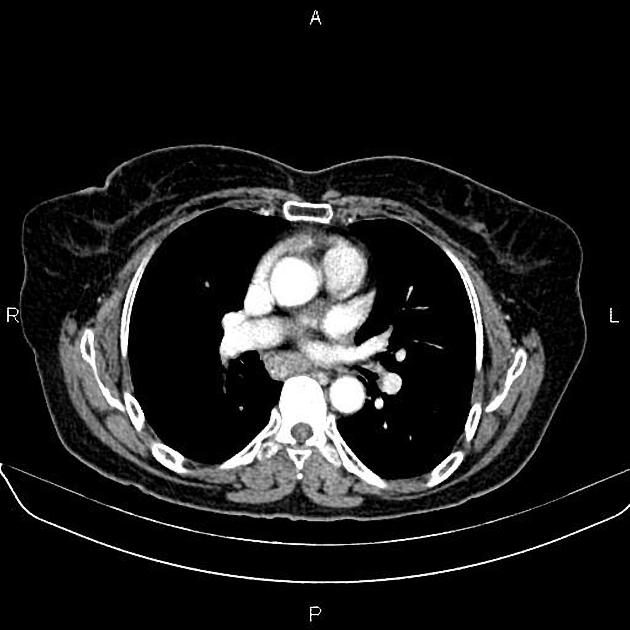
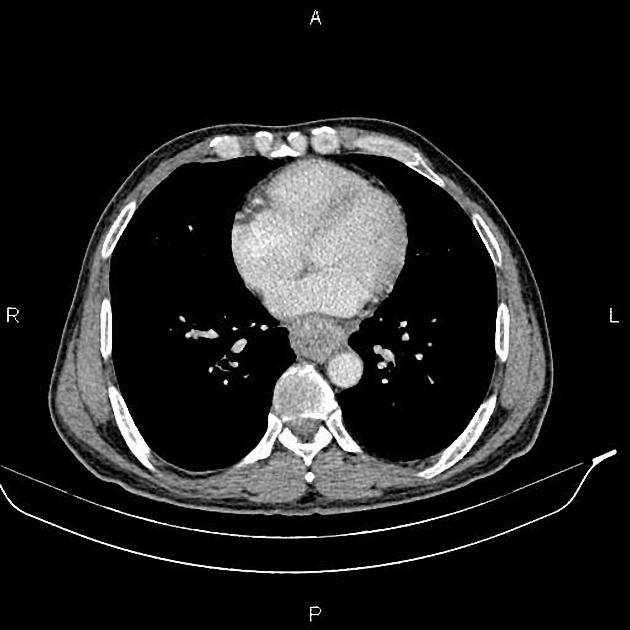
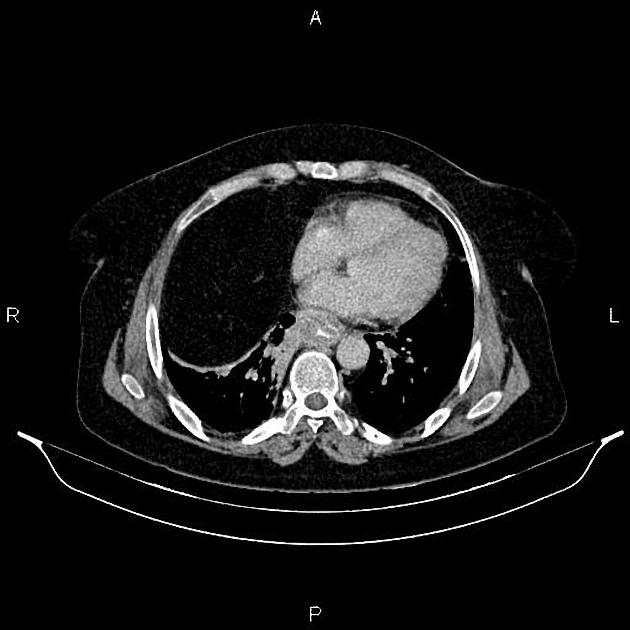
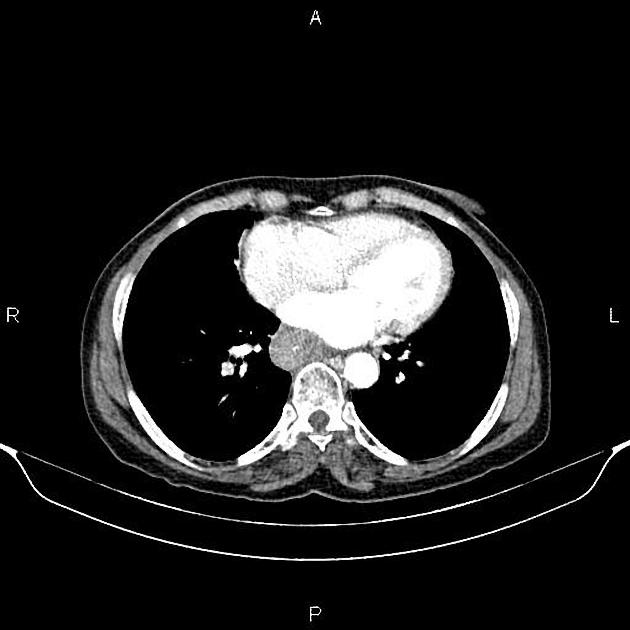
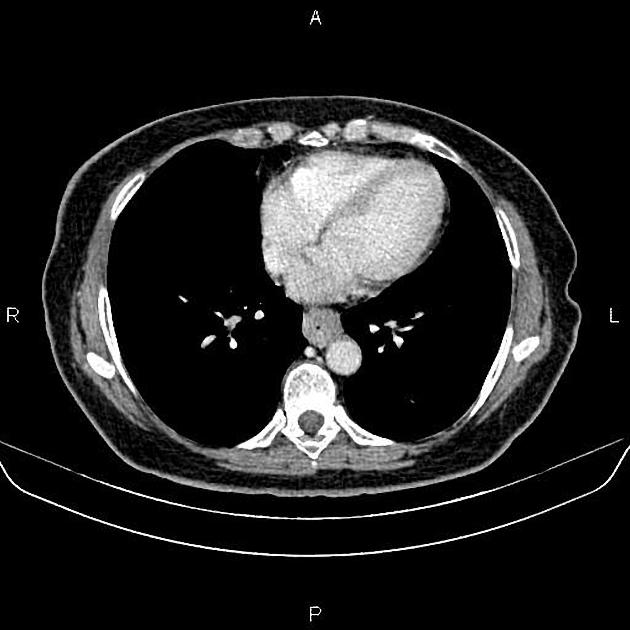
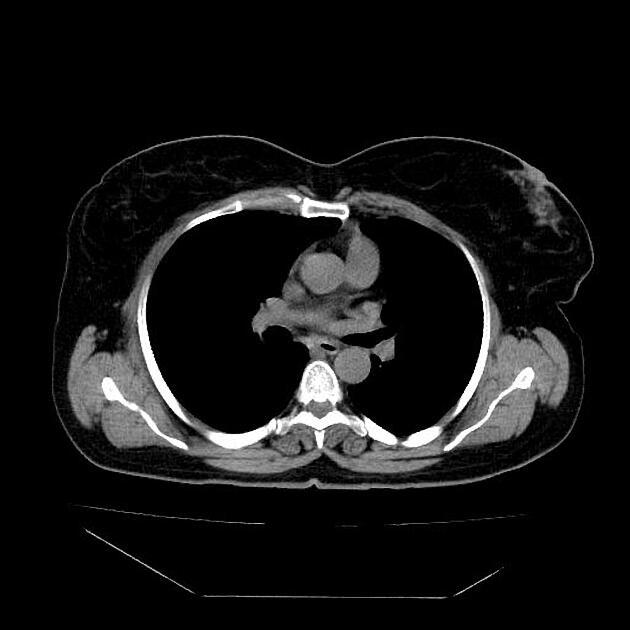
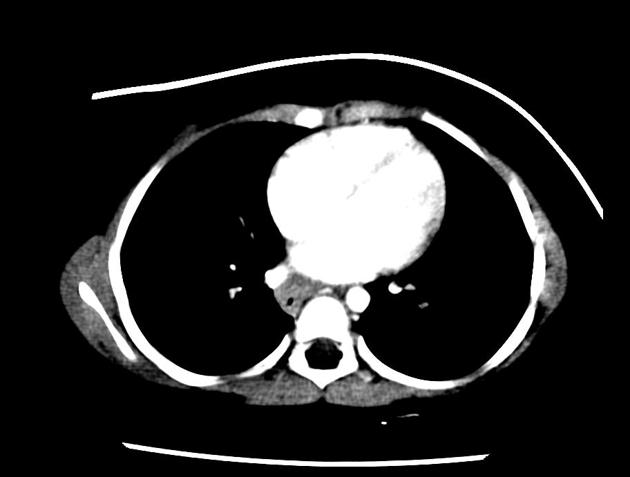
eccentric or circumferential wall thickening >5 mm
perioesophageal soft tissue and fat stranding
dilated fluid- and debris-filled oesophageal lumen is proximal to an obstructing lesion
tracheobronchial invasion appears as a displacement of the airway (usually the trachea or left mainstem bronchus) as a result of the mass effect by the oesophageal tumour
aortic invasion
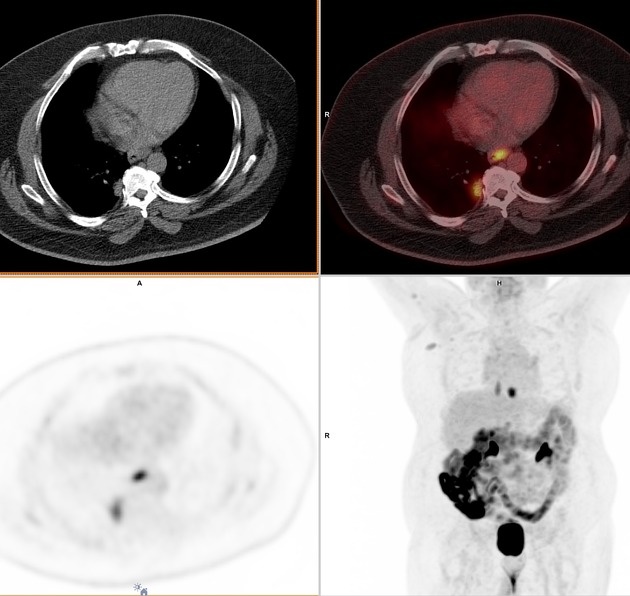
PET-CT
FDG PET-CT is useful for detecting oesophageal primary tumours. Yet, it has little role in helping determine the specific T classification because it provides limited information about the depth of tumour invasion.
PET-CT is also superior to CT for detecting lymph node metastases and can depict metastases in normal-sized lymph nodes through the uptake of FDG.
PET-CT has a primary role in depicting distant sites of metastatic disease.
The bones and liver are the most common sites of distant metastases detected at PET (but frequently missed at CT).
Treatment and prognosis
The 5-year mortality depends on the stage of the tumour. Unfortunately, most cases present with regional or distant metastatic disease (30% and 40%, respectively).
localised disease: ~40% 5-year survival
distant metastatic disease: ~5% 5-year survival
Endoscopic mucosal resection, without or with localised ablation, is an option for localised (T1a) disease. These epithelial tumours are usually <2 cm, asymptomatic, and noncircumferential.
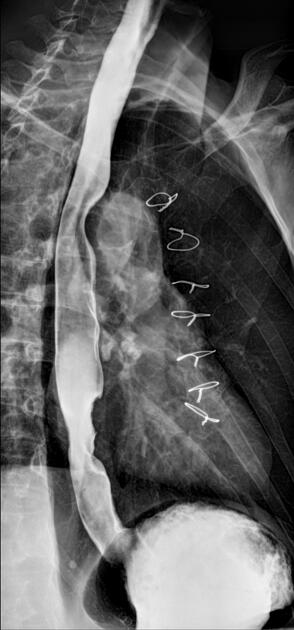
For T1b tumours and above, surgical options are mostly limited to oesophagectomy (including sometimes with palliative colonic interposition (see case 19)).
Complications
fistula formation to the trachea (5-10%), bronchi or mediastinum: can be either due to direct tumour progression or iatrogenic effects (e.g. radiation therapy)
Differential diagnosis
Imaging differential considerations include:
-
benign tumours of the oesophagus
non-malignant conditions (e.g. diffuse inflammation)









 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.