Radiation-induced pulmonary fibrosis is the late manifestation of radiation-induced lung disease and is relatively common following radiation therapy for chest wall or intrathoracic malignancies.
This article does not deal with the changes seen in the acute phase. Please refer to the article on radiation-induced lung disease for a general discussion and radiation pneumonitis for a specific discussion of acute changes.
On this page:
Epidemiology
For a discussion of the epidemiology of radiation-induced lung disease, please refer to the parent article: radiation-induced lung disease.
Clinical presentation
Radiation-induced pulmonary fibrosis is typically seen between 6 and 12 months following completion of radiation therapy course and can continue to progress for up to 2 years 1.
Although the majority of patients are asymptomatic, referred symptoms include a persistent dry cough and shortness of breath 2.
Rarely, particularly nowadays with the new techniques of radiation therapy, the radiation-induced chronic lung injury has been described to evolve to chronic respiratory failure, pulmonary hypertension, or chronic cor pulmonale 2.
Pathology
Ionizing irradiation causes damage to lung epithelium releasing inflammatory mediators that attract inflammatory cells. These in turn secrete profibrotic cytokines and chemokines, amplifying the inflammatory response. These profibrotic mediators stimulate fibroblasts to produce extracellular matrix proteins (e.g. collagen) resulting in the excess deposition of these materials 3.
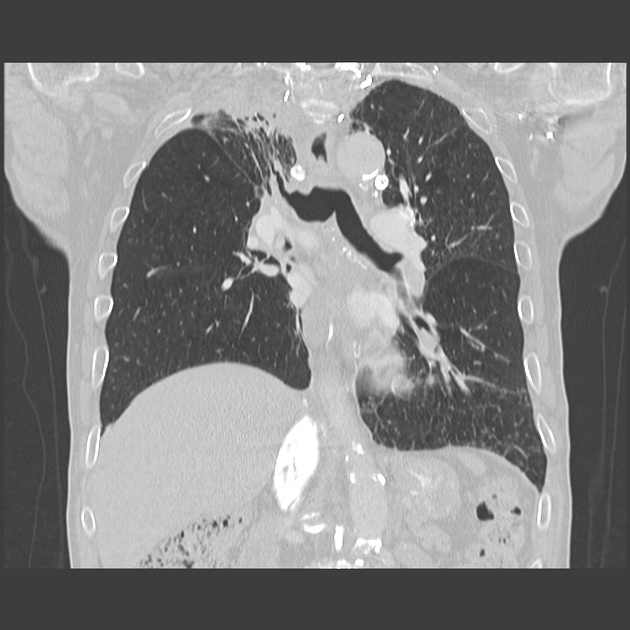
Radiographic features
Although changes in the lung are usually confined to the irradiated field, changes in the remainder of the lung may also occasionally be seen 1.
Plain radiograph
Volume loss, architectural distortion, mediastinal shift, hemidiaphragm elevation, and bronchiectasis may all be seen 2,4. In some instances, a straight edge conforming to the irradiation portal may be evident. Review of previous imaging will usually show the progression from radiation pneumonitis (hazy opacities) progressively becoming more reticular or linear with gradual loss of volume 4.
CT
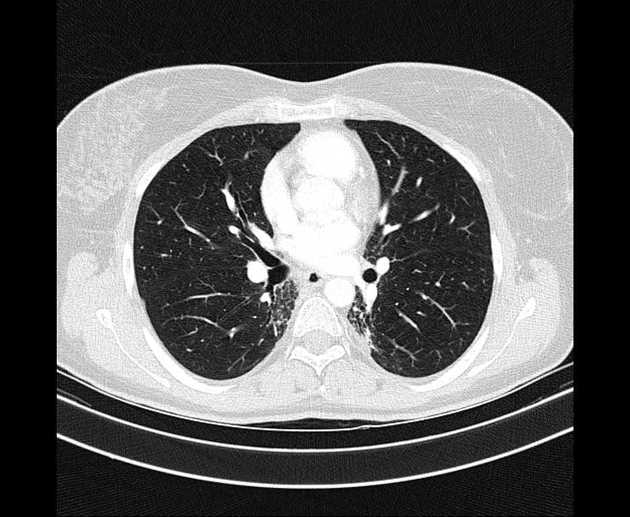
CT can better delineate parenchymal changes including volume loss and bronchiectasis, and often demonstrates the change restricted to the distribution of the irradiated field, rather than respecting anatomical boundaries (e.g. pleural fissures) 2. Changes include 1,2,4,5:
volume loss
linear scarring
-
chronic consolidation often with air bronchograms
usually having a geographic non-anatomical distribution
may cross fissures
hilar vascular displacement
mediastinal shift
pleural thickening
ipsilateral pleural effusion
Cavitation is rarely seen in the late acute phase of the radiation-induced lung injury as a consequence of pulmonary necrosis 2, and infection is a differential to consider when they are present 1,2.
It should be noted that with highly conformal radiation therapy (e.g. 3D-CRT, IMRT, SBRT, and proton therapy), the shape of the irradiated field will not have straight edges or conform to the traditional conventional radiation therapy portals. As such it may be less artificial in shape and more tightly restricted to the tumor (cf. straight lines traditionally seen in conventional radiation therapy portals) 2,6:
scar-like pattern: characterized by a band-like opacity in the tumor location associated with mild volume loss 2
mass-like pattern: characterized by a focal consolidation/ground glass in the tumor region, with or without air bronchograms or traction bronchiectasis 2
MRI
Although MRI may have a role helping to distinguish malignancy from fibrosis, care must be taken in interpreting results as, granulation tissue, edema, and areas of necrosis can all mimic tumor nodule, especially when fibrosis is also present 7.
Nuclear Medicine
PET-CT
Once the inflammation has receded, usually between 9 to 15 months after the completion date, FDG-PET is useful in differentiating radiation fibrosis from recurrent or radiation-induced malignancy, as the former will not be metabolically active 1.
Bear in mind that FDG avidity is usually present until late phases of radiation pneumonitis (3 to 9 months after treatment completion) due to the presence of residual inflammation, therefore, PET-CT is of equivocal clinical value in this period 2.
Treatment and prognosis
When fibrosis has become established, no treatment is available, other than a follow-up to assess for tumor recurrence.
Differential diagnosis
If a clear demarcation conforming to the irradiation port is seen, then there is often little difficulty in making the diagnosis, especially when a history of chest radiation therapy is known.
A knowledge of the time course of changes with respect to radiation therapy, total dose administered, administration of chemotherapy, and shape of the portal used can all have a significant impact on the differential, and thus should be sought if the referring clinician has not provided them 2. With the highly conformal radiation therapies, any further increase in size or bulkiness of the residual scar-like or mass-like patterns in the treated area is concerning for recurrent disease.
Differential includes:
other causes of pulmonary fibrosis
other causes of bronchiectasis
tuberculosis (especially in cases where the lung apices have been irradiated)
-
recurrent or radiation-induced malignancy:
in-field recurrence usually occurs within 3 years after the completion date 2
-
increase in the size of the treated area scar-like or mass-like pattern of fibrosis
bear in mind that post-radiation fibrotic changes usually occur at around 9 months but can be seen up to 2 years after treatment completion 2
malignancy often lacks air bronchograms and has convex outer borders 1,5
involvement of chest wall, bone, or lymph node increase may be present 5
-
FDG-PET-CT is useful as it will demonstrate increased metabolic activity in malignancies 1
false-positive FDG uptake in areas of inflammation may occur 2








 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.