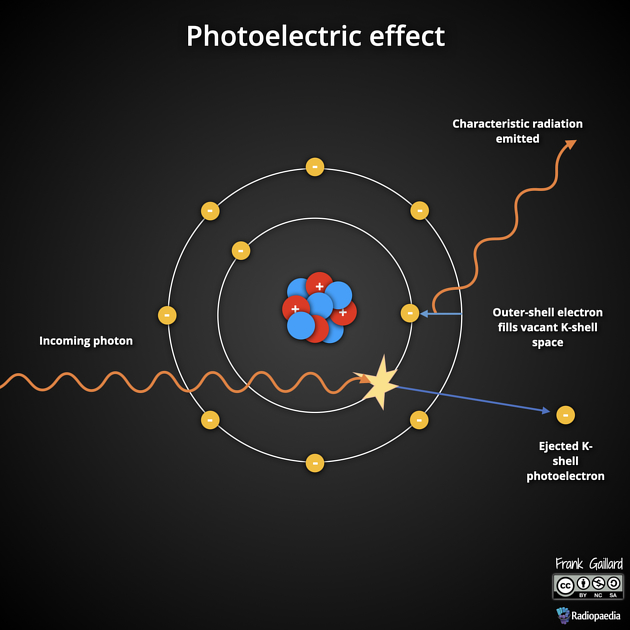
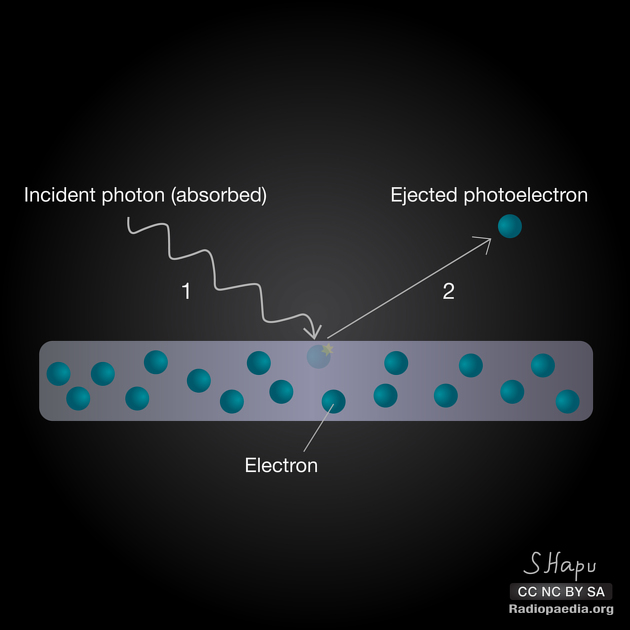
The photoelectric effect, a.k.a. photoelectric absorption, is one of the principal forms of interaction of x-ray and gamma photons with matter. A photon interacts with the inner shell electron of the atom and removes it from its shell.
On this page:
Probability of photoelectric effect
The probability of this effect is maximum when:
the energy of the incident photon is equal to or just greater than the binding energy of the electron in its shell (K-absorption edge) and
the electron is tightly bound (as in K shell) 4
The electron that is removed is then called a photoelectron and the incident photon is completely absorbed in the process. Hence, the photoelectric effect contributes to the attenuation of the x-ray beam as it passes through matter.
To stabilize the atom an outer shell electron fills the vacancy in the inner shell. The energy which is lost by this electron as it drops to the inner shell is emitted as characteristic radiation (an x-ray photon) or as an Auger electron.
The probability of photoelectric absorption occurring is
proportional to the cube of atomic number of the attenuating medium (Z), and
inversely proportional to the cube of the energy of the incident photon (E), and
proportional to the physical density of the attenuating medium (p)
The probability of photoelectric effect rapidly approaches zero at incident photon beam energy of 140keV in water 4.
Thus the overall the probability of photoelectric absorption can be summarized as follows:
Photoelectric absorption ~ p·(Z³/E³)
Therefore if Z doubles, photoelectric absorption will increase by a factor of 8 (2³ = 8), and if E doubles photoelectric absorption will reduce by a factor of 8. Small changes in Z and E can therefore significantly affect photoelectric absorption.
Applications
At low photon energies, photoelectric effect dominates in lead. This phenomenon holds true until 0.511 MeV when Compton effect starts to predominate the photoelectric effect in radiation attenuation 5. This has practical implications in the field of radiation protection and is the reason why materials with a high Z such as lead (Z = 82) are useful shielding materials 3.
Photoelectric absorption is also utilized in mammography and when using contrast agents to improve image contrast. The dependence of photoelectric absorption on Z and E means that it is the major contributor to beam attenuation up to approximately 30 keV when human tissues (Z = 7.4) are irradiated. At beam energies above this, the Compton effect predominates.
History and etymology
It was Philipp Eduard Anton von Lenard, awarded the Nobel Prize for Physics in 1905, among the first to carry out systematic studies of the photoelectric effect taking up and extending Herz's research (Lenard's trigger hypothesis): light only triggers the release of selected electrons; it doesn't add energy to them (1902) 8.
Albert Einstein received the Nobel Prize in 1921 for the correct interpretation of the phenomenon even if he actually withdrew it - due to a series of controversies - a year later, in 1922. The quantization of energy is therefore inherent in the intimate structure same as light, which travels in the form of energy packets that act like particles and which have since been called photons 6,7.




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.