Solitary fibrous tumors of the dura are rare dural masses, histologically identical to solitary fibrous tumors found elsewhere and encompassing hemangiopericytomas (previously thought of as separate diagnoses). This was reflected in the 4th edition WHO classification of CNS tumors (2016).
Solitary fibrous tumors are composed of spindle cells thought to be of mesenchymal origin. They usually occur in middle-aged individuals and symptoms depend on the size of the mass and its location.
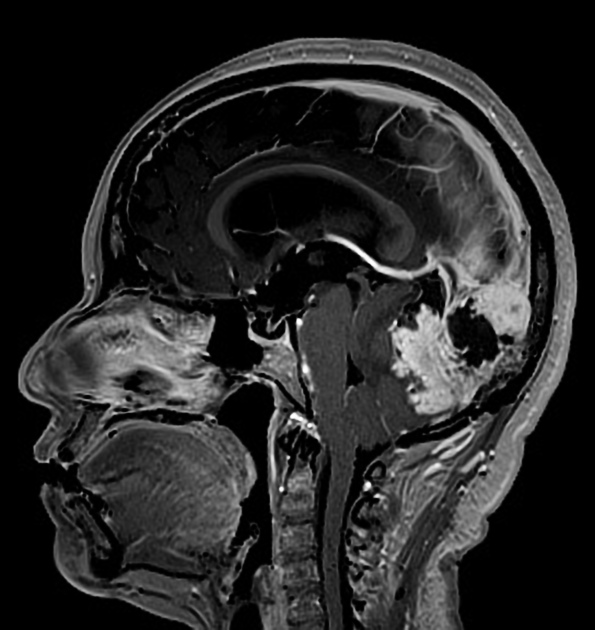
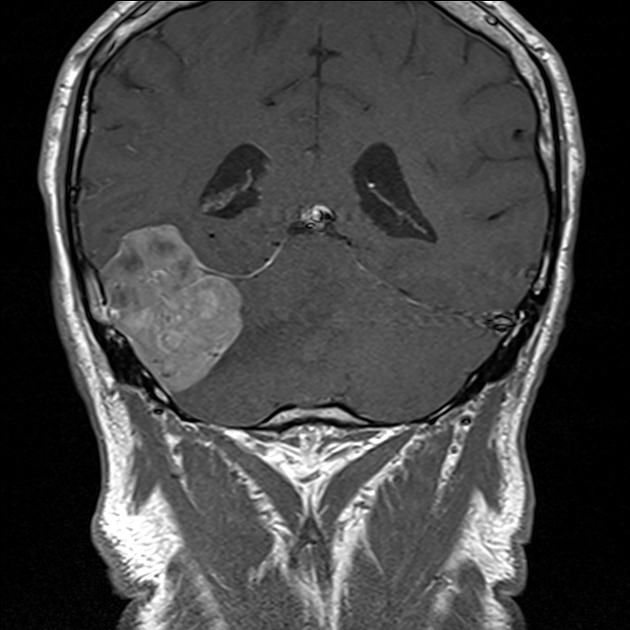
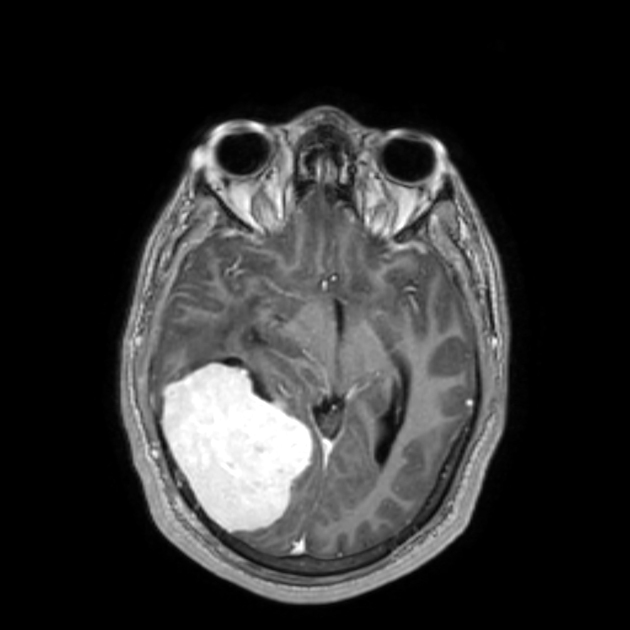
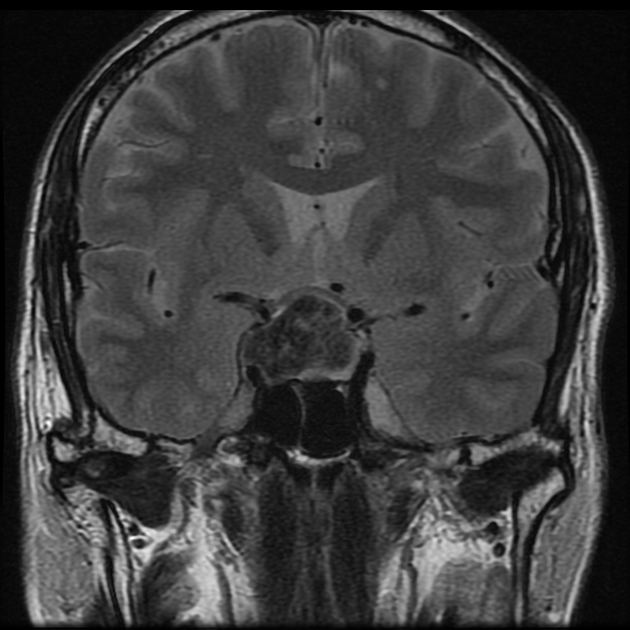
On imaging, these tumors are similar to meningiomas, appearing as extra-axial well-circumscribed solid masses with vivid contrast enhancement.
On this page:
Epidemiology
Solitary fibrous tumors occur in older middle-aged individuals (mean age: 47-56 years), without a convincing gender predilection, although some reports have found increased incidence in females 1,2.
Clinical presentation
Clinical presentation is nonspecific and identical to other dural masses (e.g. meningioma). Symptoms depend on the size of the mass and its location. Headache is the most common presenting complaint 1.
Rarely, solitary fibrous tumors result in non-islet cell tumor hypoglycemia (NICTH) as a result of secretion of insulin-like growth factor-2 (IGF-2) 3. Rarer still is acromegaly 3.
Pathology
Grading
Although this is an area of some debate with alternative grading systems having been proposed, usually, solitary fibrous tumors of the dura can be graded from WHO grade 1 to grade 3; what was previously known as hemangiopericytomas representing WHO grade 2 or 3 tumors) 5,9.
Macroscopic appearance
These are well-circumscribed and firm 5.
Microscopic appearance
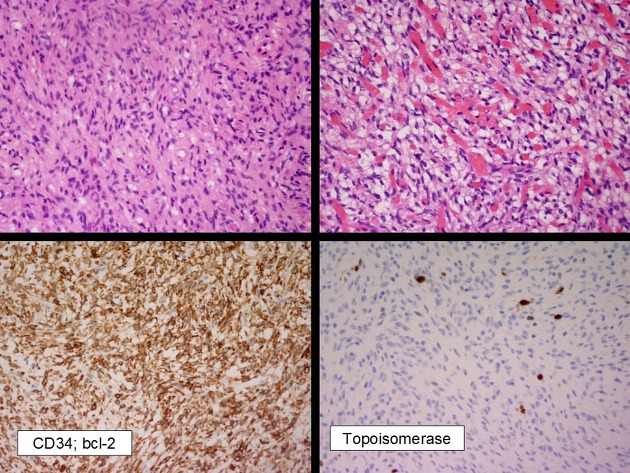
Typically, solitary fibrous tumors demonstrate areas thick bands of collagen separating areas of hypercellularity and hypocellularity. Staghorn vessels, classically seen in hemangiopericytomas, are also commonly seen 5.
Immunophenotype
The immunohistochemical appearance of solitary fibrous tumors is characterized by 1,2,5:
CD34: positive
vimentin: positive
STAT6 protein: relocation of this protein to the nucleus
EMA: usually negative
PS100: negative
Genotype
genomic inversion of 12q13 locus resulting in the fusion of NAB2 and STAT6 genes 5
Radiographic features
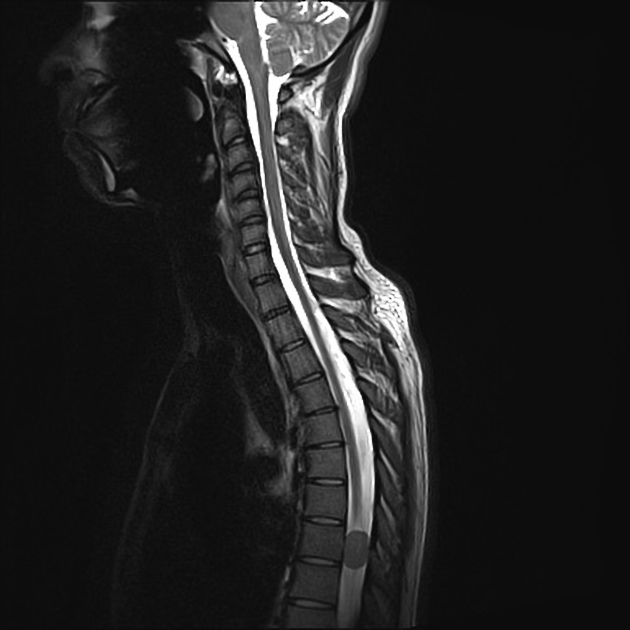
The radiographic appearances are those of a well-circumscribed mass arising from the dura (supratentorial convexity, falx and tentorium and posterior fossa). Unlike solitary fibrous tumors of the spinal cord, which most frequently arise from the cord itself and not the theca 2, parenchymal tumors of the brain are very rare.
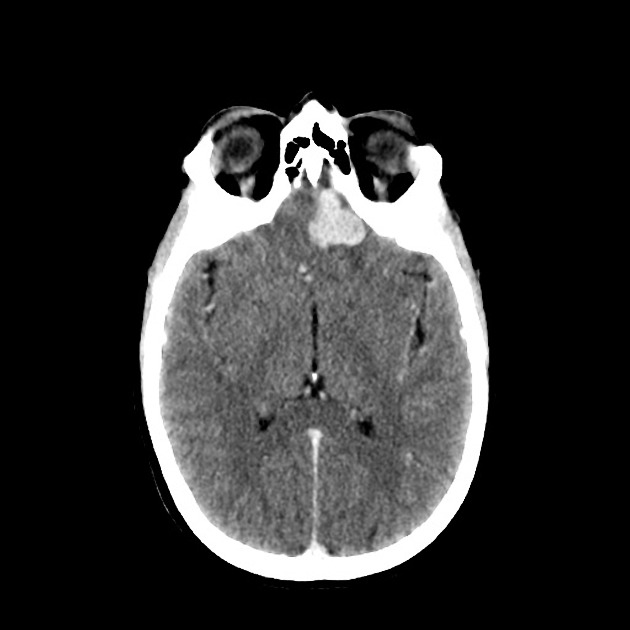
CT
Solitary fibrous tumors are well-circumscribed and isodense to hyperdense compared to adjacent brain. They may demonstrate calcifications and may erode bone if abutting it 1.
MRI
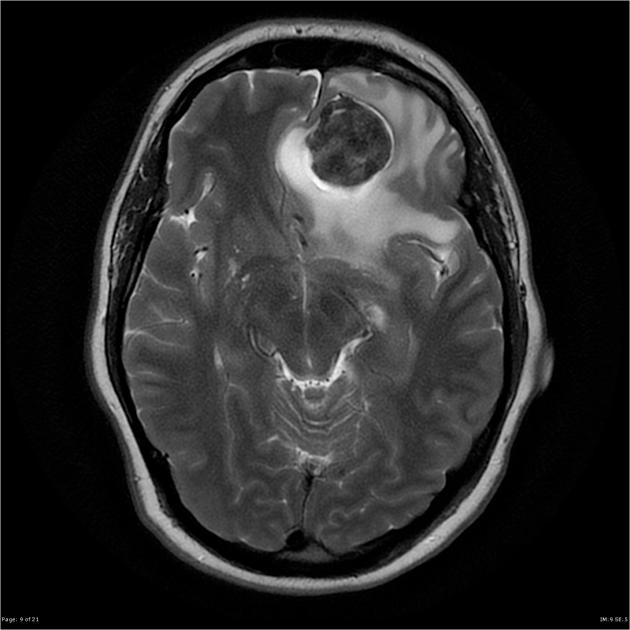
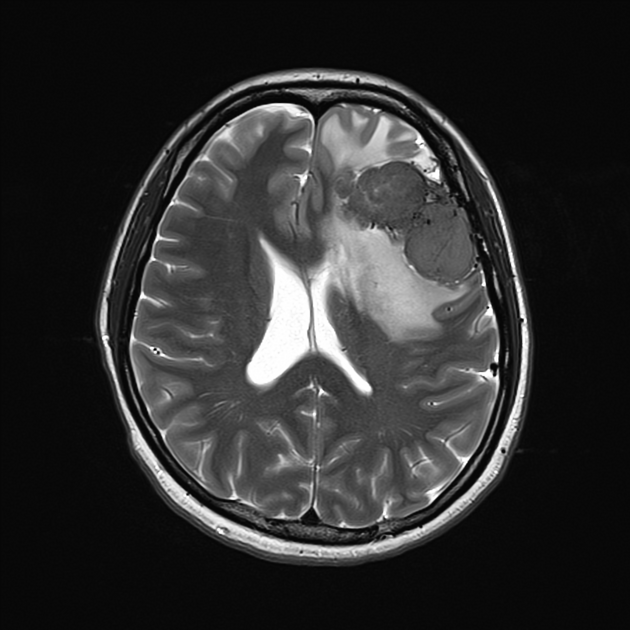
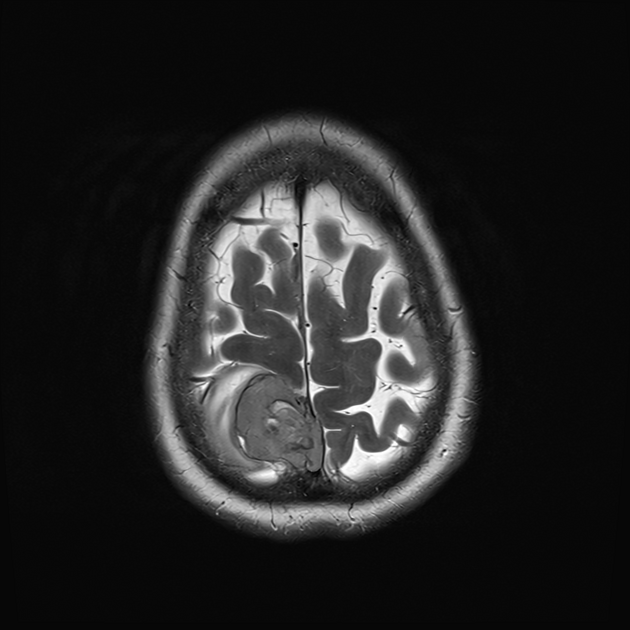
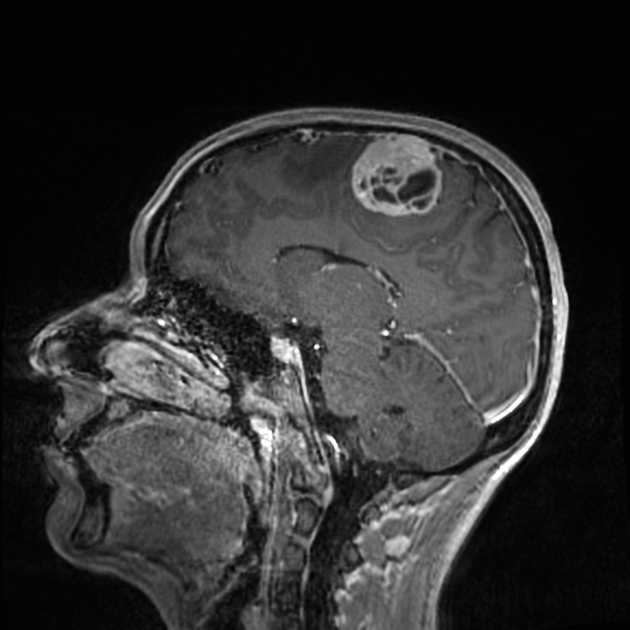
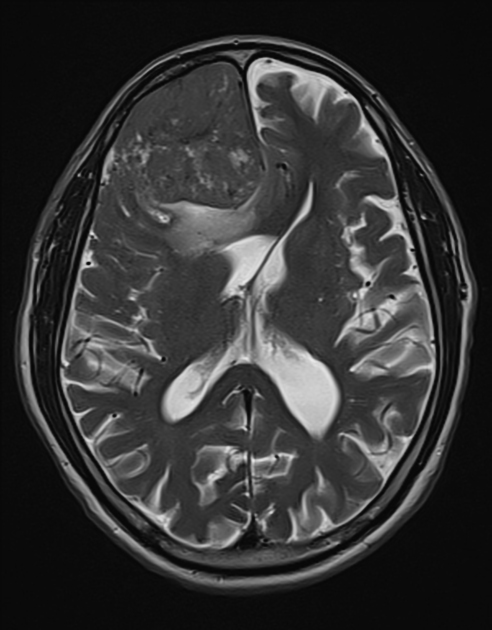
These tumors are similar to meningiomas, appearing as extra-axial well-circumscribed masses, with the following signal characteristics 1,2:
-
T1
typically, intermediate signal similar to brain
-
T2
iso- to hypointensity (the best clue to the diagnosis)
heterogeneous signal: "yin-yang" appearance of separate areas with low signal and high signal intensity may be characteristic 6-8
flow voids commonly seen
-
T1 C+
vivid diffuse heterogeneous contrast enhancement of low T2 signal components
dural tail may be seen, but is much less common than in meningiomas
DWI/ADC: regions of restricted diffusion are frequently seen
-
MRS
lipid and lactate elevation
myo-inositol elevation
MR perfusion: elevated rCBV
Angiography (DSA)
Prominent tumor blush, with feeding vessels varying, depending on the location of the tumor. Importantly, in addition to meningeal supply, these tumors can receive blood supply from pial vessels 1.
Treatment and prognosis
Surgical resection, if complete, is usually curative. Recurrence rates are variably reported as low to relatively high; up to 50% in one series 2. If incomplete or recurrence occurs, then radiotherapy may be employed 1.
Differential diagnosis
The differential of solitary fibrous tumors of the dura is essentially that of other dural masses, and common entities to be considered include 1,2:
-
T2 signal iso- to hyperintense
MRS: alanine and glutamate-glutamic acid peaks may be seen
-
characteristic locations
T2 signal usually iso- to hyperintense
The most useful feature that helps in making a preoperative diagnosis is the propensity for low grade solitary fibrous tumor to be low signal on T2.
Importantly, lymphoma, Erdheim-Chester disease and melanocytic lesions may also have low T2 signal. Meningiomas may also be low on T2, usually in the setting of mineralization/calcification; thus reviewing other sequences (T2*) or CT is useful.




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.