Cerebral vasospasm following subarachnoid hemorrhage is a major complication of subarachnoid hemorrhage (SAH). It is overtaking rebleed as the major cause of mortality and morbidity in the subgroup of patients with SAH who reach the hospital and receive medical care. It usually occurs after a few days from the onset of hemorrhage and peaks in severity on days 4-7.
On this page:
Epidemiology
Cerebral vasospasm on imaging is more common in aneurysmal SAH (~50%; range 30-70%) than after traumatic brain injury, i.e. traumatic SAH (~20%; range 10-30%) or blast-related injury 1,12. It becomes clinically apparent in ~25% of patients, typically from the 4th to 10th day post bleed 1.
Clinical presentation
Vasospasms can be clinically silent. However, symptomatic vasospasm is defined as new focal neurological symptoms or deterioration of the level of consciousness attributable to vasospasm-induced ischemia after other etiologies have been ruled out 2. As such, symptomatic vasospasm is often referred to as 'delayed cerebral ischemia' in the medical literature 2, although delayed cerebral ischemia likely has a more complex pathogenesis than just vasospasm 14.
Pathology
After decades of research, the exact mechanism(s) responsible remains elusive 12 although a number of candidate agents are demonstrated to play a role. These include:
nitrous oxide (NO)
endothelin (ET) 1
oxyhemoglobin (oxyHb)
-
others:
thrombin
serotonin (5-HT)
thromboxane A2 (TXA2)
norepinephrine (NA)
sphingosine-1-phosphate
Most likely the 'true' pathway involves multiple agents interacting with each other, both biochemically and via changes in gene expression, accounting for the delay of onset.
Oxyhemoglobin, highest in concentration in arterial blood, appears to simultaneously up-regulate the expression of endothelin 1 (ET-1) and reduce the efficacy of NO.
This results in alteration of normal vascular tone, resulting in narrowing of the large vessels. Increasingly it is also becoming apparent that small caliber vessels which are in contact with blood in CSF are also narrowed - down to 15 micrometers - far too small to be visible on angiography, let alone CTA/MRA.
The result, if severe enough, is to reduce perfusion of brain parenchyma resulting in ischemic symptoms, infarction, and its sequelae.
The degree of vasospasm is difficult to predict but correlates with the original Fisher scale and more accurately with the modified Fisher scale. Hence, its likelihood and severity are associated with the amount of blood.
Radiographic features
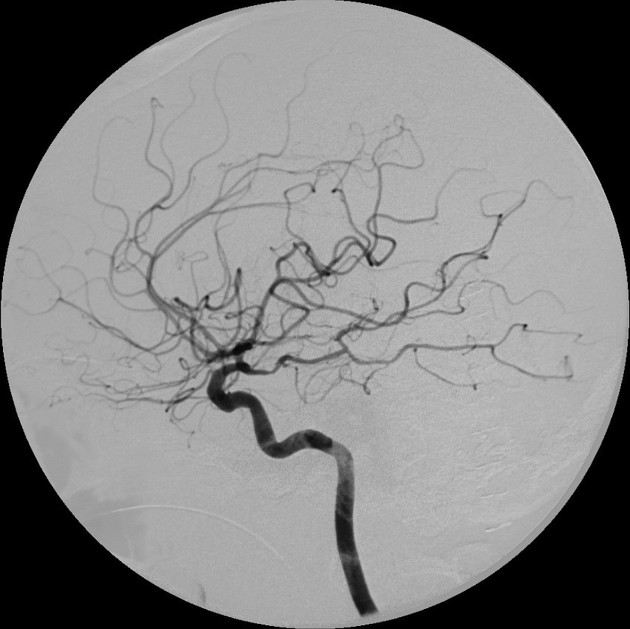
Vasospasm associated with subarachnoid hemorrhage is usually characterized by diffuse narrowing without intervening regions of normal vessel caliber 10 and can be assessed using CTA, MRA or catheter angiography. The secondary effects of vasospasm (e.g. delayed cerebral ischemia) can be imaged with CT and MR and is discussed separately.
Vascular imaging
Cerebral vasospasm appears as a smooth, relatively long segment of narrowing with tapering towards non-affected segments. It is often centered at the arterial bifurcation but does not narrow the bifurcation itself as much as adjacent segments of artery, creating the appearance of an enlarged bifurcation 10.
CT
In addition to imaging vascular stenosis directly with CTA (see above) CT perfusion can be used identify vasospasm with fairly high sensitivity (85.6%) and specificity (87.9%) 15.
Ultrasound
Transcranial Doppler (TCD) is used as a screening modality for vasospasm after SAH; it relies on the use of pulsed wave Doppler to determine the velocity of blood flow in the middle cerebral artery (MCA). The following have been suggested to be supportive of the presence of vasospasm in a suggestive clinical context 11:
middle cerebral artery mean flow velocities (MFV) >120 cm/s
-
increase in MCA MFV >21 cm/sec/day from previously measured baseline
within 3 days status post-SAH
Furthermore, TCD may be used to differentiate increased flow due to circulatory hyperdynamic states using the Lindegaard ratio, which is calculated by taking the product of the MCA and ipsilateral internal carotid artery mean flow velocities. Ratios over 3.0 are suggestive that vasospasm is responsible for the elevated flow velocity.
Treatment and prognosis
Aggressive, early and prophylactic treatment can markedly reduce the incidence of vasospasm but often requires early securing of the ruptured aneurysm. Three main modalities are employed:
-
triple H therapy
traditionally, Haemodilution, Hypertension, Hypervolaemia to maintain adequate cerebral perfusion pressure was advocated with hydration and inotropes
however, given that hypervolemia has demonstrated potential for harm, euvolaemia is generally advocated instead of hypervolemia 13
-
calcium channel blockers
nimodipine is the most widely used calcium channel blocker in this setting, which dilates vessels, especially leptomeningeal collaterals, and prevents vasospasm 13
-
endovascular intervention
-
intra-arterial vasodilators (spasmolysis) 13
intra-arterial delivery of a calcium channel blocker such as nimodipine or verapamil has replaced previously used drugs such as papaverine
they are administered by slow bolus injection into the relevant vascular territory via a standard diagnostic catheter, with careful monitoring of blood pressure
treatment may need to be repeated daily for 3-5 days
-
a more invasive neurointerventional technique requiring a guiding catheter and placement of an endovascular microballoon over a guidewire across the affected segment
expanding the balloon disrupts the smooth muscle fibers within the vessel wall
there is a risk of vessel dissection or rupture
once treated the spasm does not usually recur
-
Despite treatment, approximately half of all symptomatic patients will show severe permanent neurological deficits or will die 1.









 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.