Glioblastomas (GBM) are the most common adult primary brain tumor and are aggressive, relatively resistant to therapy, and have a corresponding poor prognosis.
They typically appear as heterogeneous masses centered in the white matter with irregular peripheral enhancement, central necrosis, and surrounding vasogenic edema, although molecular glioblastomas may appear indistinguishable from lower-grade astrocytoma, IDH-mutant.
Treatment primarily consists of surgery with concurrent radiotherapy and temozolomide.
On this page:
Terminology
Since 1926 when the term "glioblastoma multiforme" was coined, the definition of this tumor has substantially changed, particularly over the past decade with an increasing reliance on molecular markers to define these tumors.
IDH-wildtype
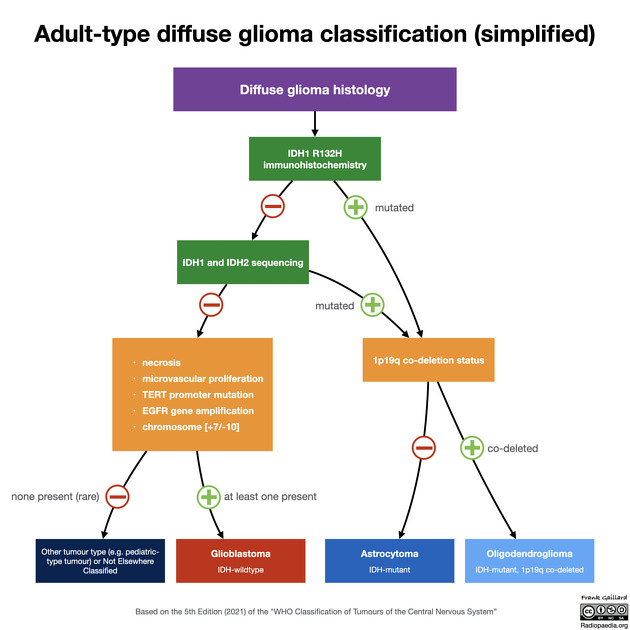
In the 5th edition (2021) of the WHO classification of CNS tumors, glioblastomas have been defined as diffuse astrocytic tumors in adults that must be IDH-wildtype, and are now an entirely separate diagnosis from astrocytoma, IDH-mutant grade 2, 3 or 4 5.
Multiforme
Glioblastoma was previously known as glioblastoma multiforme; the multiforme referred to the tumor heterogeneity. In the revised 4th edition (2016) of the WHO classification, the term "multiforme" was dropped, with these tumors referred to merely as glioblastomas. In the revised 4th edition, the abbreviation GBM was kept for disambiguation 16 however it appears to have been deprecated in the 5th edition summary 20.
Primary and secondary
Glioblastomas had traditionally been divided into primary and secondary; the former arising de novo (90%) and the latter developing from a pre-existing lower grade tumor (10%).
These historical terms now correlate closely to IDH-mutation status but should no longer be used.
Primary glioblastomas largely equate to glioblastoma, IDH-wildtype, whereas secondary glioblastomas now equate to astrocytoma, IDH-mutant, WHO CNS grade 4.
Variants
In the 5th edition (2021) WHO classification of CNS tumors, three glioblastoma histological variants are recognized (which are discussed separately), as well as a number of histological patterns which are discussed below 16.
The three recognized variants are:
Epidemiology
Glioblastomas, now defined as IDH-wildtype tumors, are essentially tumors of adults, usually occurring after the age of 40 years with a peak incidence between 65 and 75 years of age. There is a slight male preponderance with a 3:2 M:F ratio 5. White patients are affected more frequently than other ethnicities: the prevalence in Europe and North America is 3-4 per 100,000, whereas in Asia it is 0.59 per 100,000 16.
The vast majority of glioblastomas are sporadic. Rarely they are related to prior radiation exposure (radiation-induced glioma). They can also occur as part of rare inherited tumor syndromes, such as p53 mutation-related syndromes including neurofibromatosis type 1 (NF1) and Li-Fraumeni syndrome. Other syndromes in which glioblastomas are encountered include Turcot syndrome, Ollier disease, and Maffucci syndrome.
Clinical presentation
Typically patients present in one of three ways:
focal neurological deficit
symptoms of increased intracranial pressure
seizures
Rarely (<2%) intratumoral hemorrhage occurs and patients may present acutely with stroke-like symptoms and signs.
Diagnosis
The 5th edition (2021) of the WHO classification of CNS tumors incorporates molecular parameters into the diagnostic criteria. In this classification, to make the diagnosis of a glioblastoma the following are required 20:
adult patient
diffuse astrocytic tumor
IDH-wildtype *
-
and at least one of the following:
necrosis
microvascular proliferation
TERT promoter mutation
EGFR gene amplification
combined gain of whole chromosome 7 and loss of chromosome 10 [+7/-10]
In the rare situation where these criteria are not met, it is likely the tumor will be denoted as not elsewhere classified (NEC) although a variety of pediatric-type diffuse gliomas may be worth considering 20.
* An IDH wild-type status can be reached without the need for sequencing in patients over the age of 55 years on the basis of negative IDH-1 R132H immunohistochemistry, as the likelihood of finding other IDH mutations in older age is very unlikely 20.
Pathology
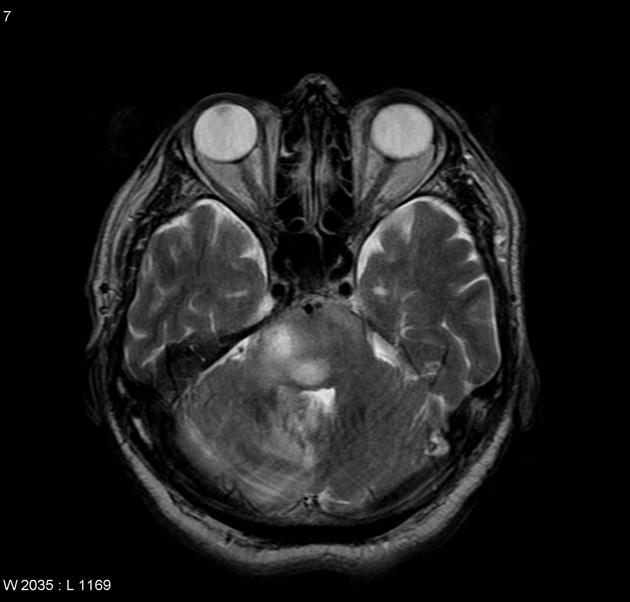
Although glioblastomas can arise anywhere within the brain, they have a predilection for the subcortical white matter and deep grey matter of the cerebral hemispheres, particularly the temporal lobe 16.
Macroscopic appearance
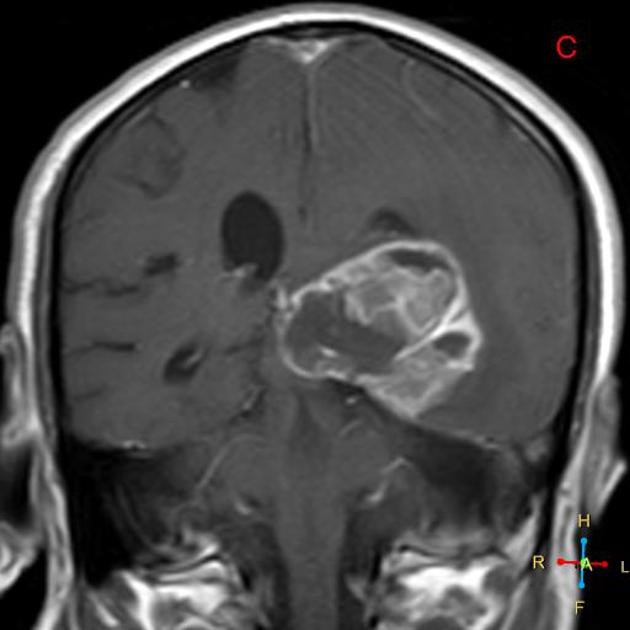
Glioblastomas are typically poorly marginated, diffusely infiltrating, necrotic masses localized to the cerebral hemispheres. The supratentorial white matter is the most common location.
These tumors may be firm or gelatinous. Considerable regional variation in appearance is characteristic. Some areas are firm and white, some are soft and yellow (secondary to necrosis), and others are cystic with local hemorrhage. Glioblastomas have significant variability in size from only a few centimeters to lesions that replace a hemisphere. Infiltration beyond the visible tumor margin is always present.
These tumors are multifocal in 20% of patients but are rarely truly multicentric. They may also demonstrate a gliomatosis cerebri growth pattern.
Microscopic appearance
Histologically, pleomorphic astrocytes with marked atypia and numerous mitoses are seen. Necrosis and microvascular proliferation are hallmarks of glioblastomas.
Areas of necrosis are typically surrounded by elongated cells that are arranged in parallel to each other, orthogonal to the necrotic center; this is known as pseudopalisading and is fairly unique to glioblastoma 2,20,27.
Microvascular proliferation results in an abundance of new vessels with a poorly formed blood-brain barrier (BBB) permitting the leakage of iodinated CT contrast and gadolinium into the adjacent extracellular interstitium resulting in the observed enhancement on CT and MRI respectively 11.
Edema and enhancement are however also seen in lower grade tumors that lack endovascular proliferation (such as diffuse astrocytomas, IDH-mutant) and this is thought to be due to disruption of the normal blood-brain barrier by tumor produced factors. Vascular endothelial growth factor (VEGF) for example has been shown to both disrupt tight junctions between endothelial cells and increase the formation of fenestrations 12.
Cellular variants
Glioblastomas are capable of demonstrating varied patterns, sometimes within one tumor. In addition to giant cell glioblastoma, gliosarcoma, and epithelioid glioblastoma, other histological features are sometimes encountered which impact imaging appearance and biological behavior. These include 16:
-
gemistocytes
more commonly seen in grade 4 astrocytomas
-
granular cells
histologically mimic macrophages and thus can lead to a misdiagnosis of macrophage-rich demyelination
lipidized cells
-
metaplasia
most commonly squamous epithelium
if dominant feature then a diagnosis of gliosarcoma should be considered
-
multinucleated giant cells
a common feature of glioblastoma
if they are the dominant feature then a diagnosis of giant cell glioblastoma should be considered
-
primitive neuronal cells
previously known as glioblastoma with PNET-like component
more frequently has CSF spread
MYC or MYCN amplification common
IDH-mutant in 15-20% of cases
-
small cell glioblastoma
histologically appears similar to oligodendroglioma, but usually demonstrate EGFR amplification
like oligodendrogliomas, they have a predilection for extensive cortical involvement
Immunophenotype
GFAP: positive but of variable intensity
S100: positive
nestin: positive
p53 protein: positive if TP53 mutated
EGFR: positive in 40-98% of cases 16
IDH-1 R132H: negative (by definition, otherwise not an IDH-wildtype glioblastoma, but rather an astrocytoma, IDH-mutant WHO CNS grade 4) 16
H3 K27M mutation: negative (if positive then diffuse midline glioma H3 K27-altered)
Genetics
combined gain of whole chromosome 7, loss of chromosome 10 [+7/-10]
alterations of the CDK4/6–RB1 cell-cycle pathway: 80% due to deletions of CDKN2A 20
Radiographic features
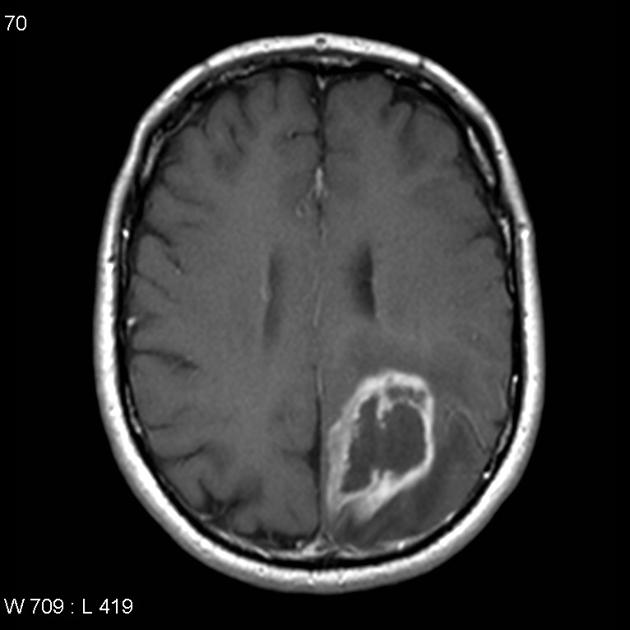
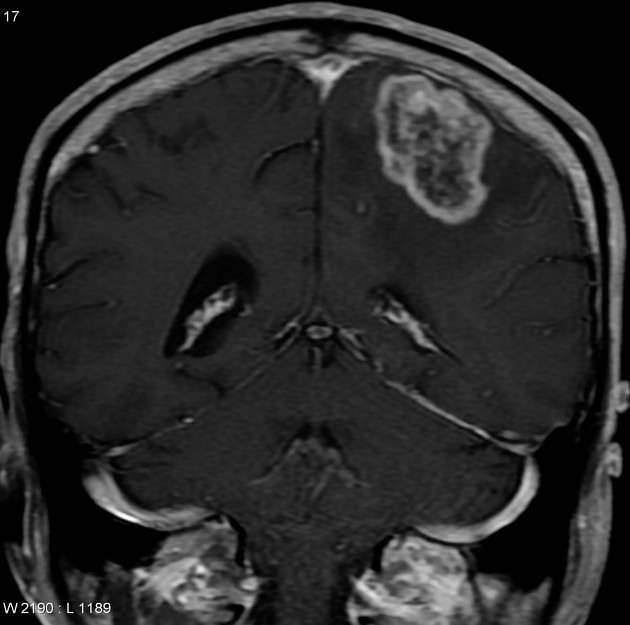
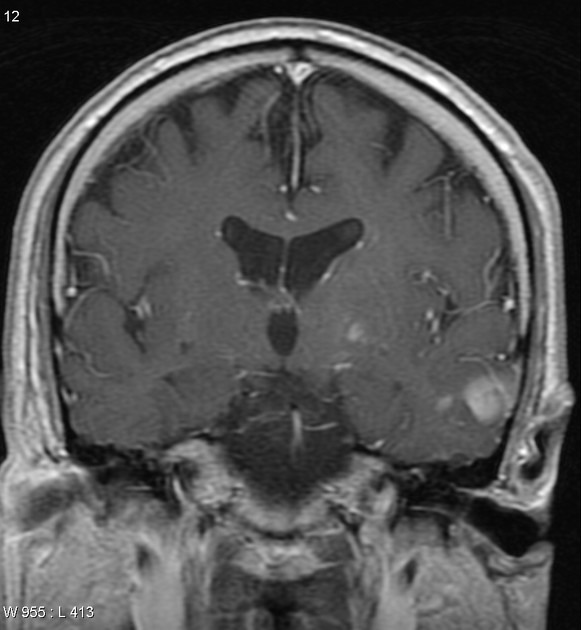
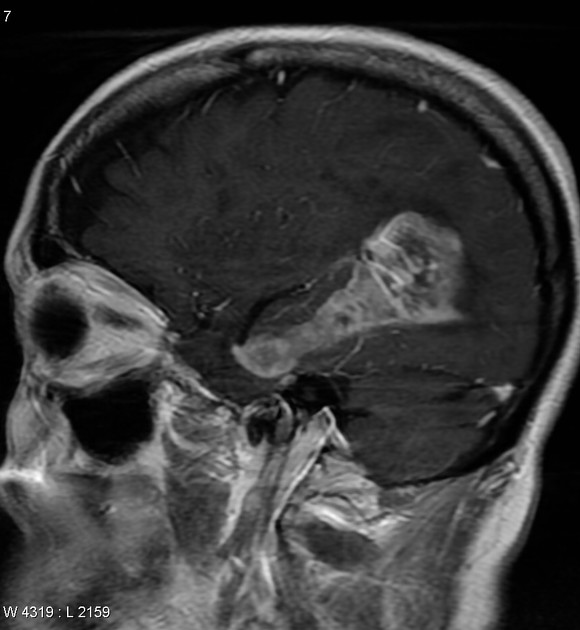
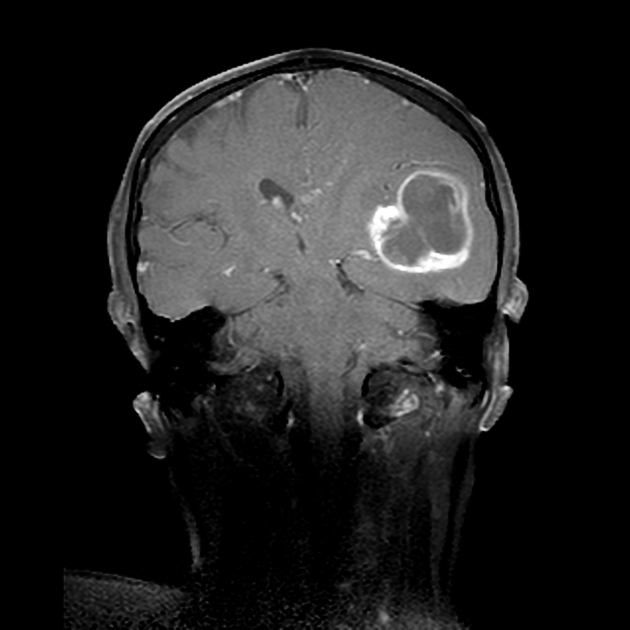
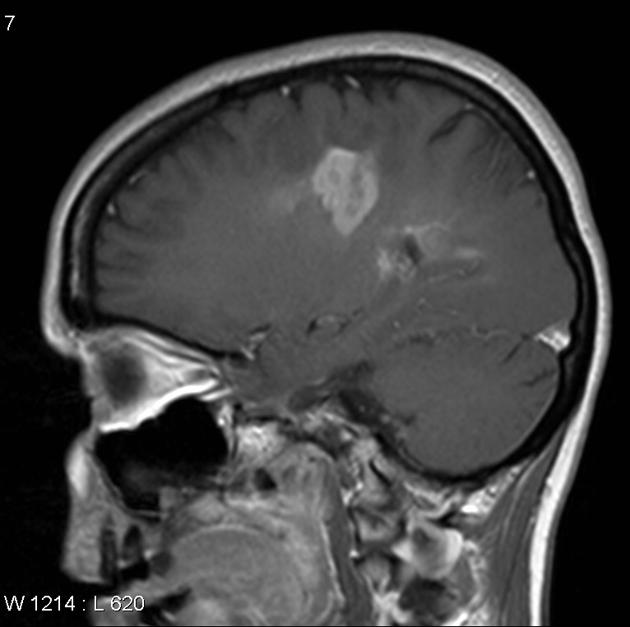
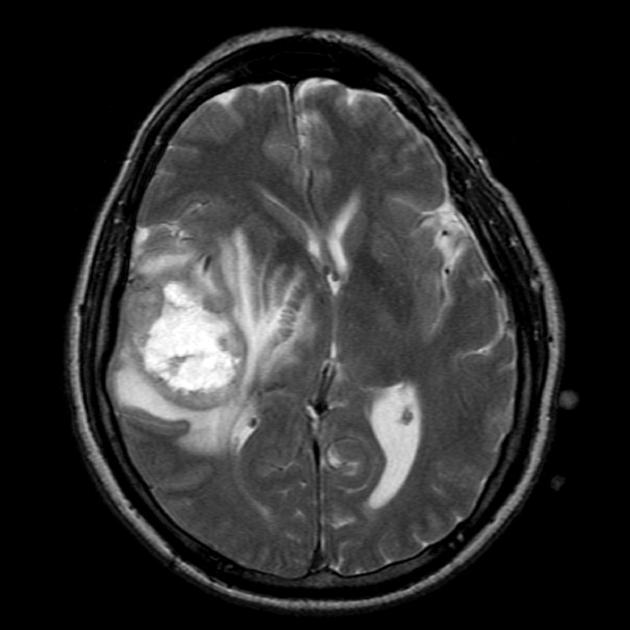
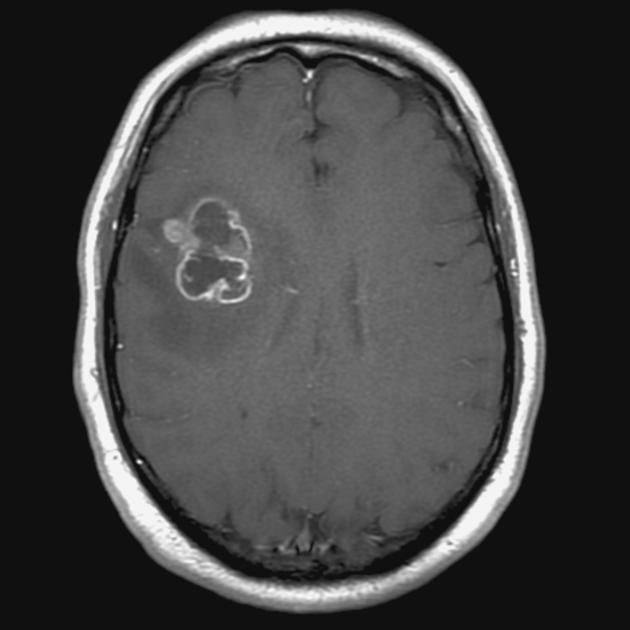
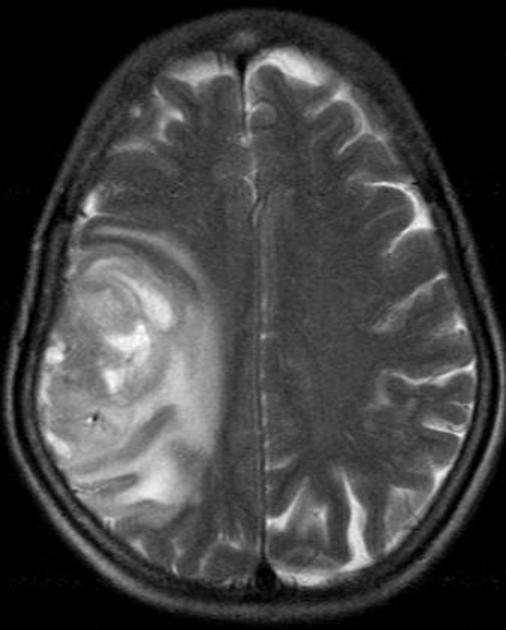
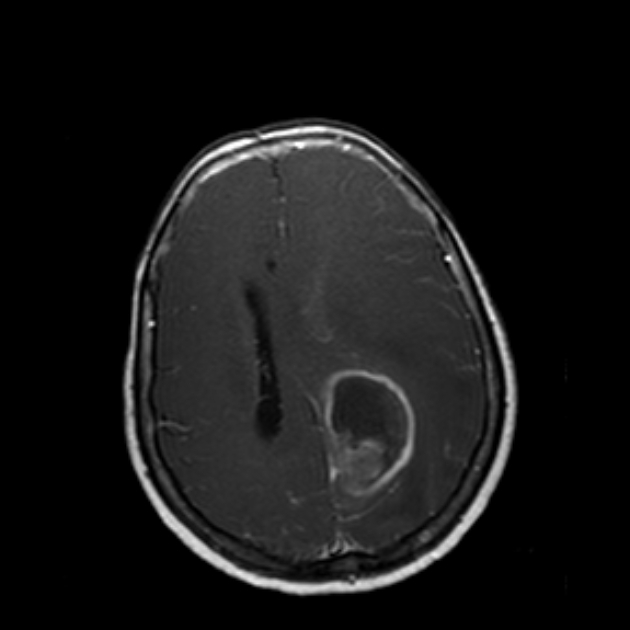
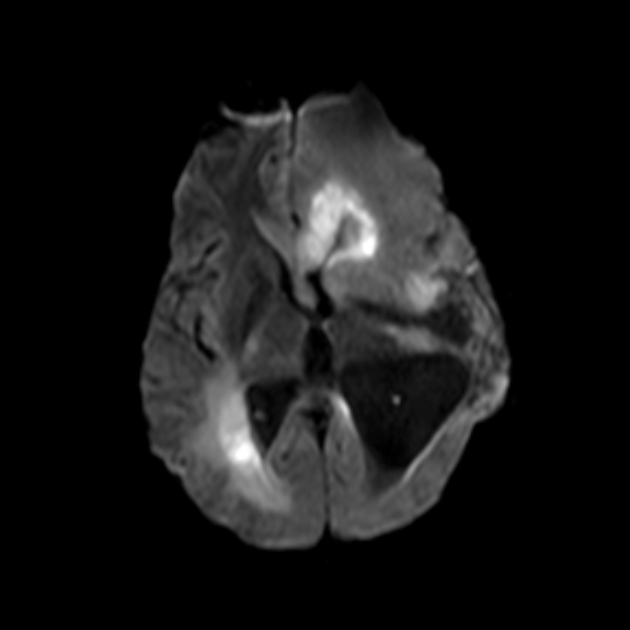
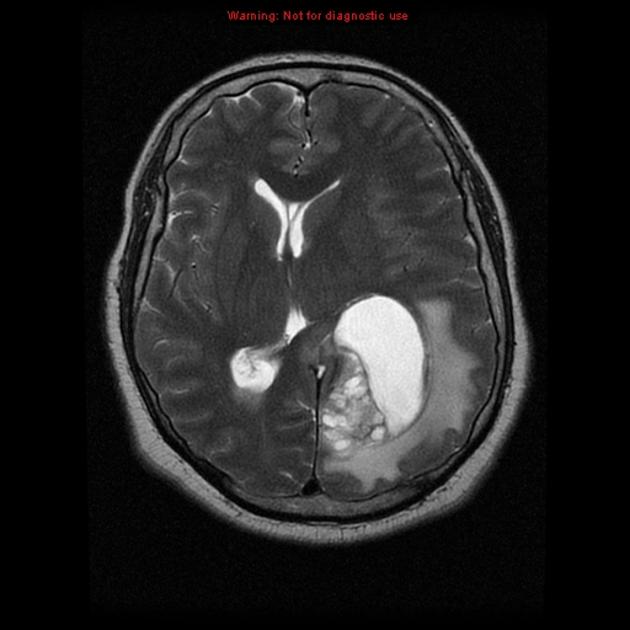
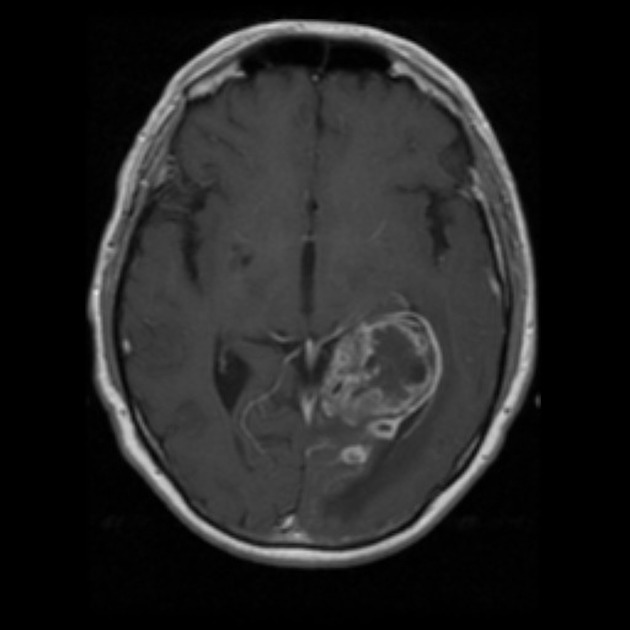
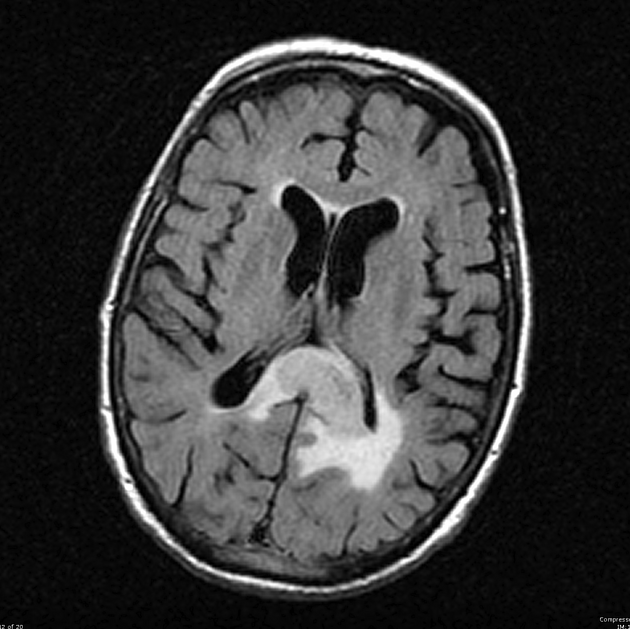
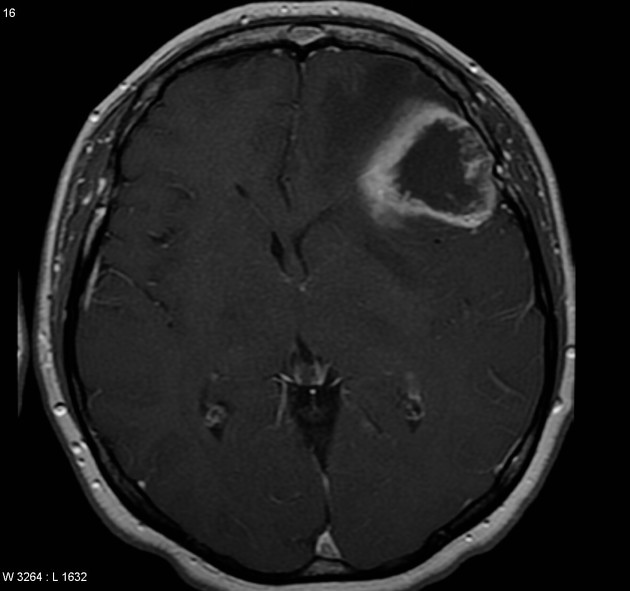
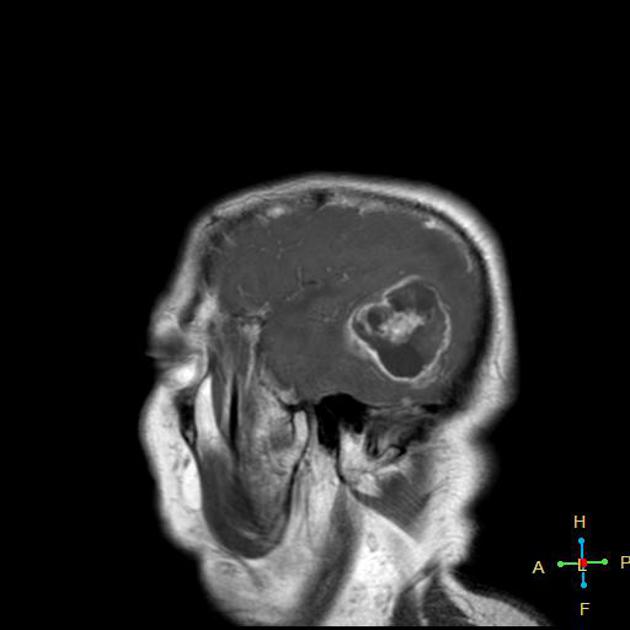
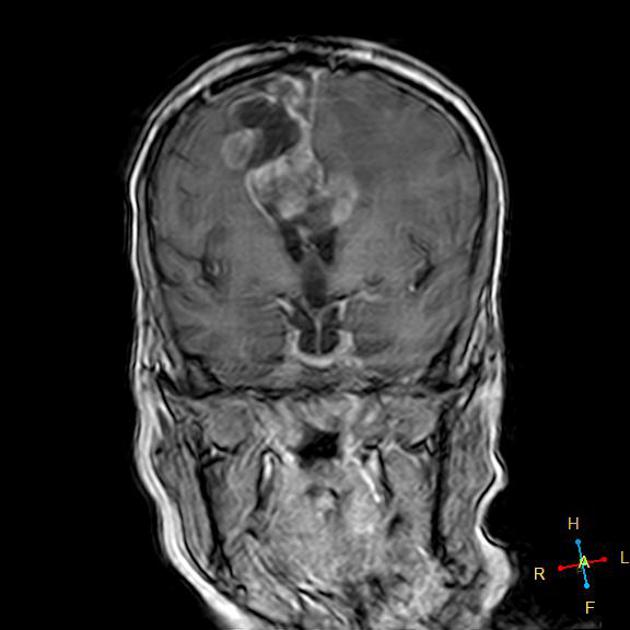
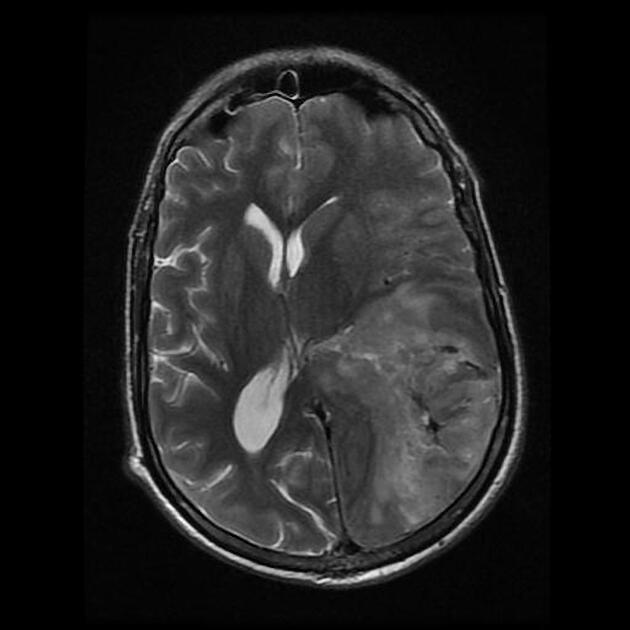
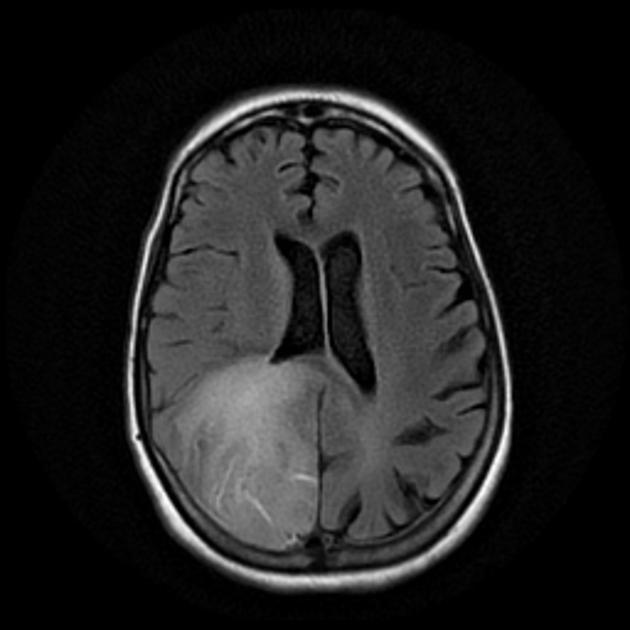
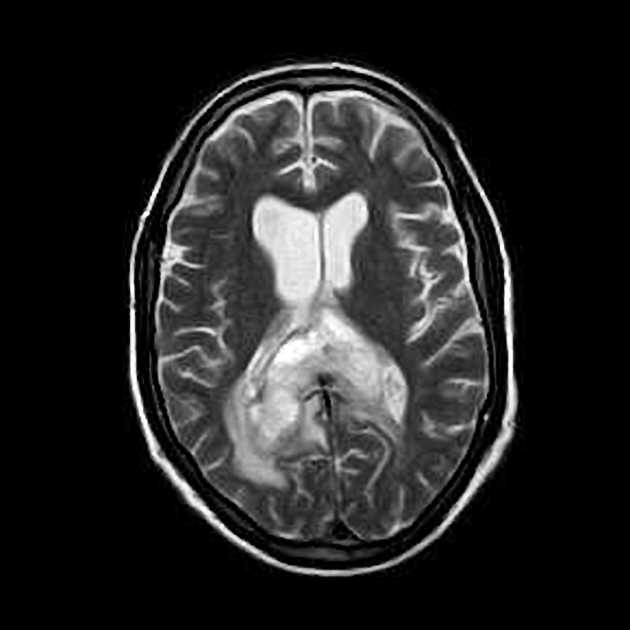
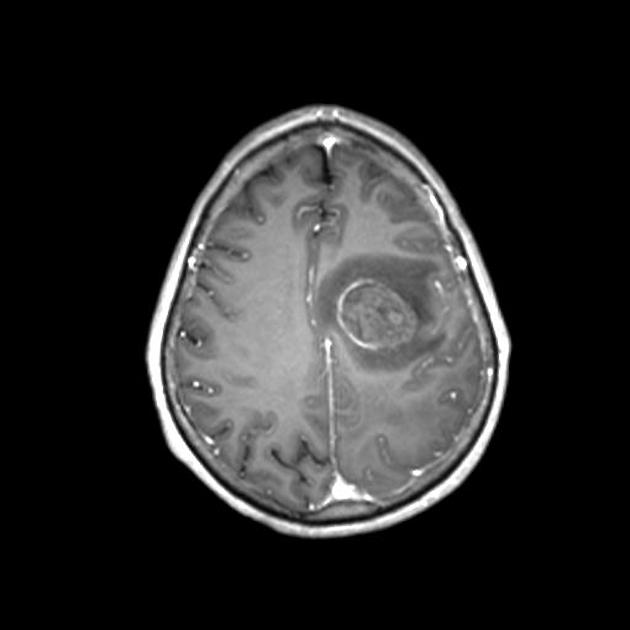
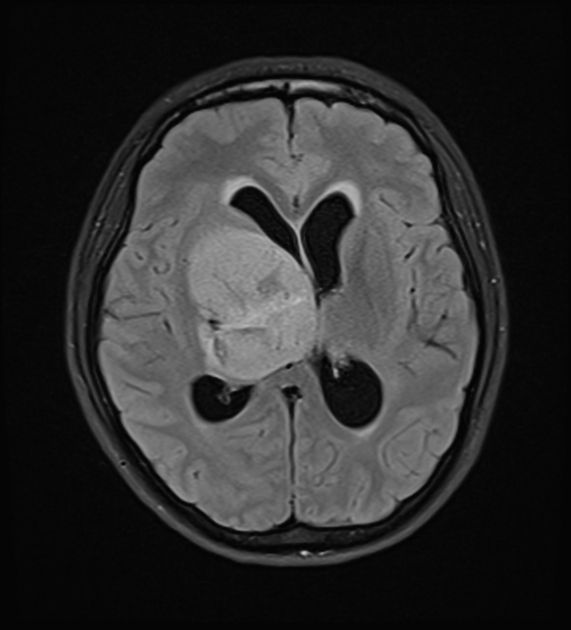
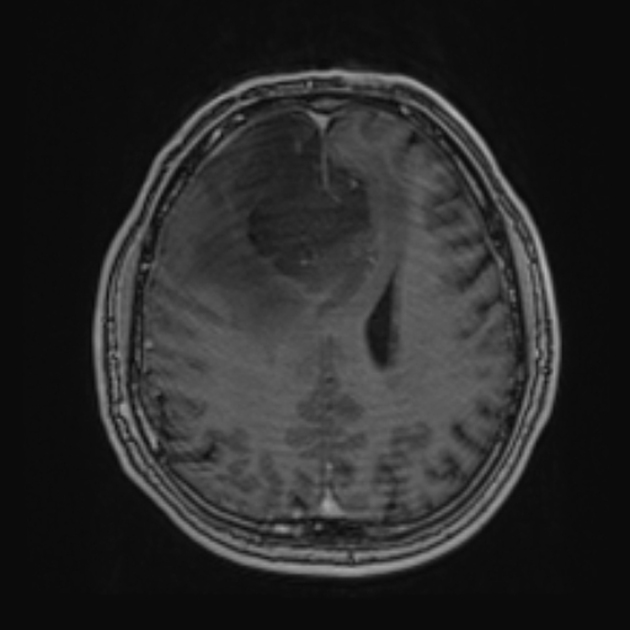
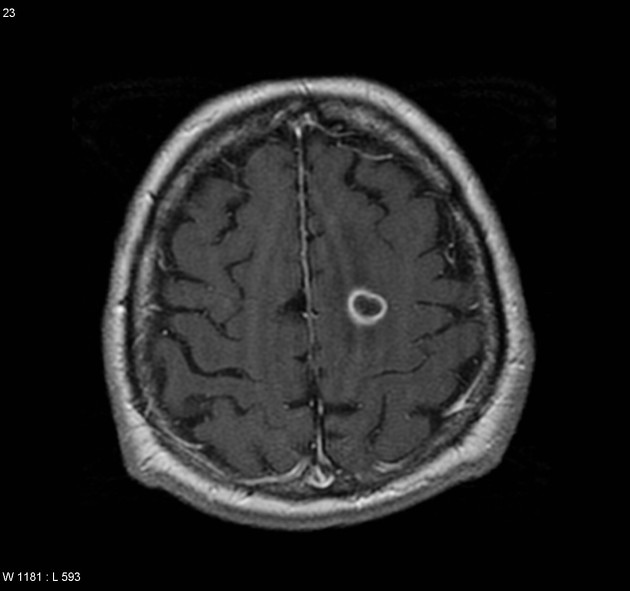
Glioblastomas are typically large tumors at diagnosis. They often have thick, irregularly enhancing margins and a central necrotic core, which may also have a hemorrhagic component. They are surrounded by vasogenic-type edema, which in fact usually contains infiltration by neoplastic cells.
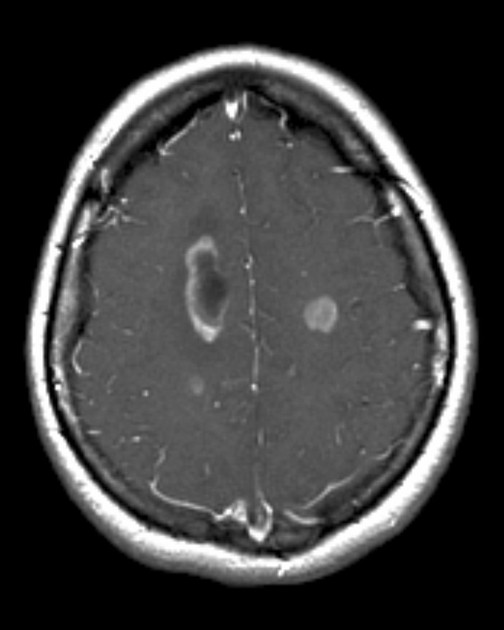
Multifocal disease, which is found in ~20% of cases, is where multiple areas of enhancement are connected to each other by abnormal white matter signal, which represents microscopic spread to tumor cells. Multicentric disease, on the other hand, is where no such connection can be seen.
It is important to note that molecular glioblastoma will have imaging features similar to, if not indistinguishable from, low grade astrocytoma, IDH-mutant.
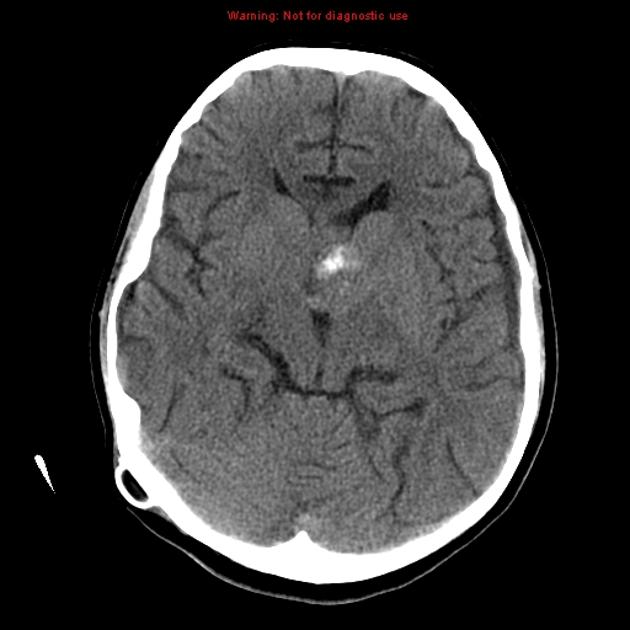
CT
irregular thick margins: iso- to slightly hyperattenuating (high cellularity)
irregular hypodense center representing necrosis
marked mass effect
surrounding vasogenic edema
hemorrhage is occasionally seen
calcification is uncommon
intense irregular, heterogeneous enhancement of the margins is almost always present
MRI
-
T1
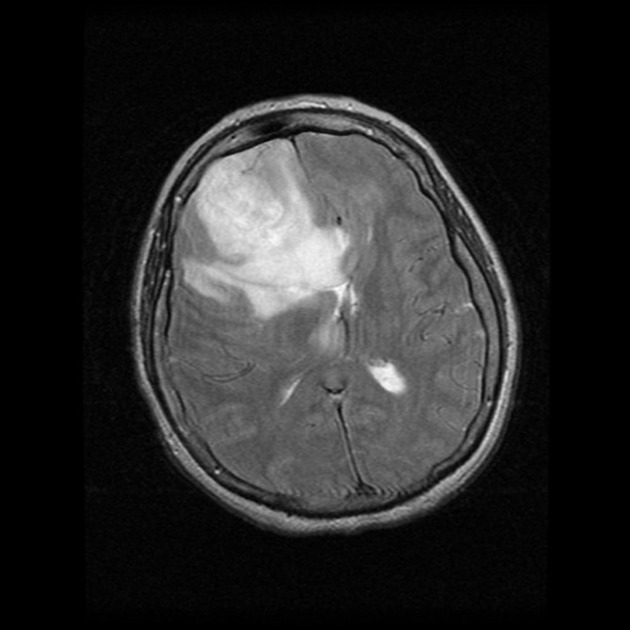
hypo to isointense mass within the white matter
central heterogeneous signal (necrosis, intratumoral hemorrhage)
-
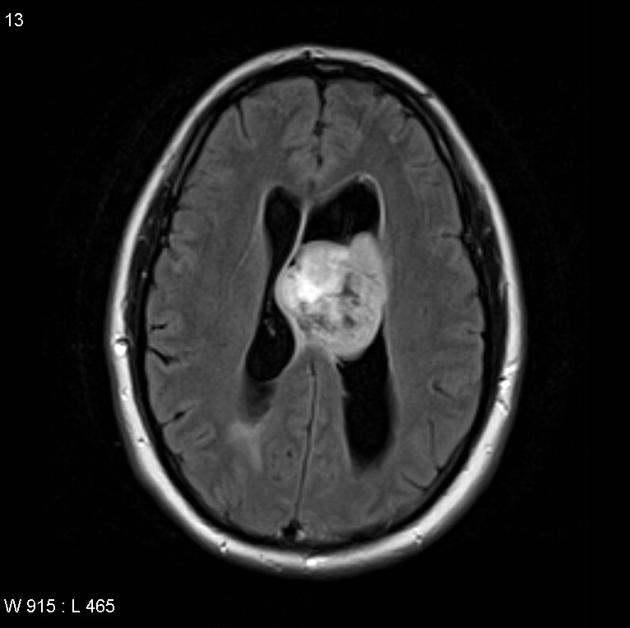
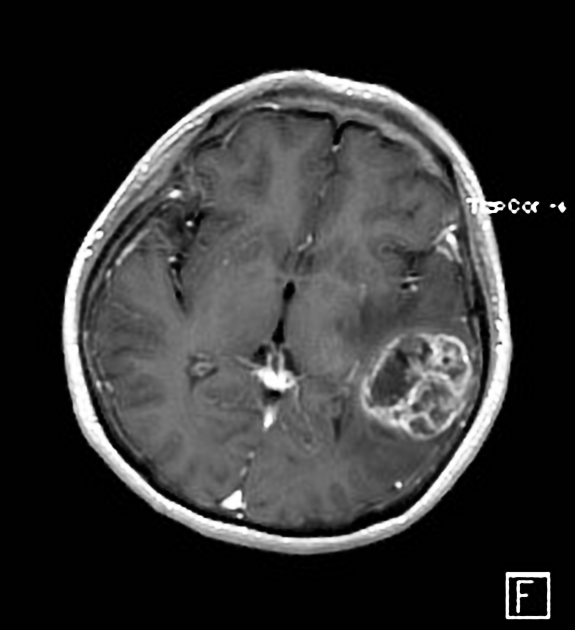
T1 C+ (Gd)
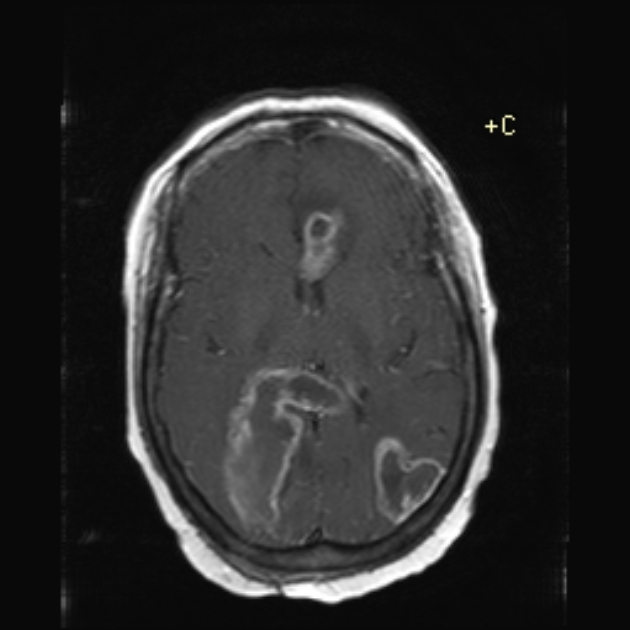
enhancement is variable but is almost always present
typically peripheral and irregular with nodular components
usually surrounds necrosis
-
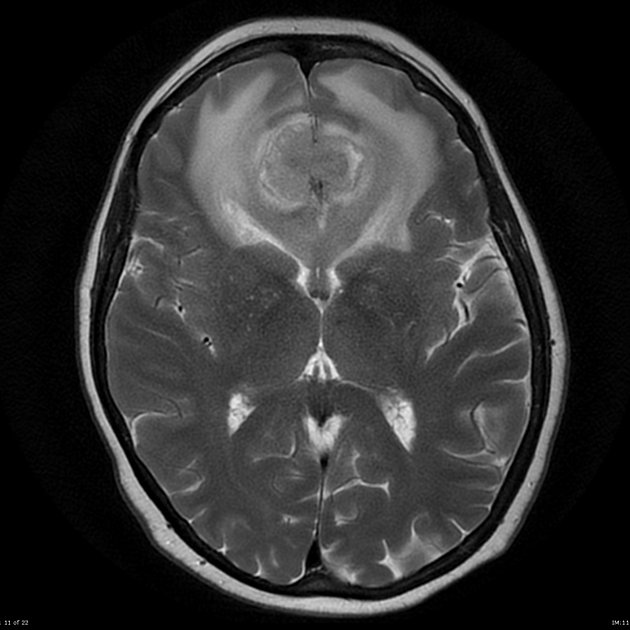
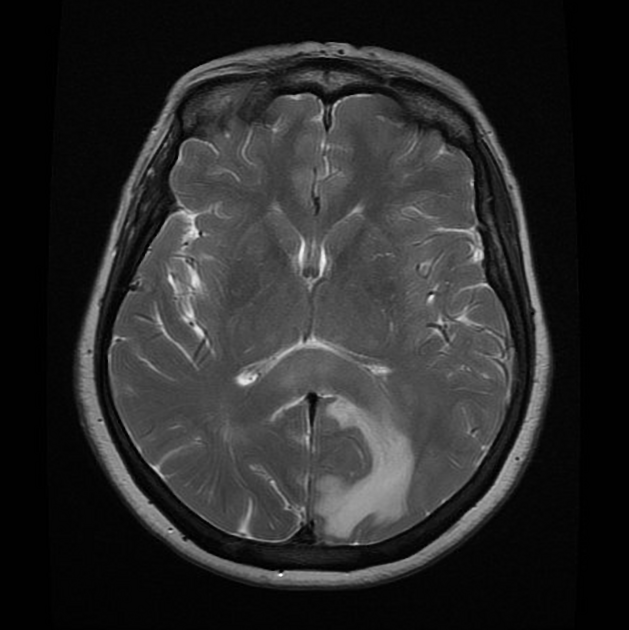
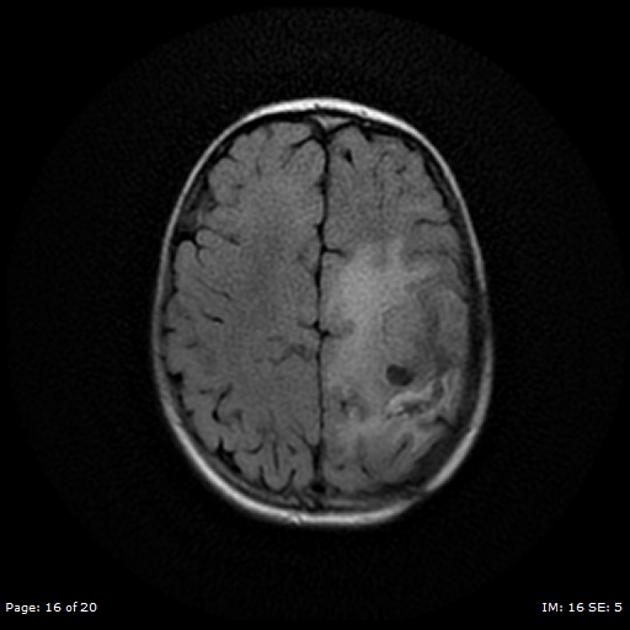
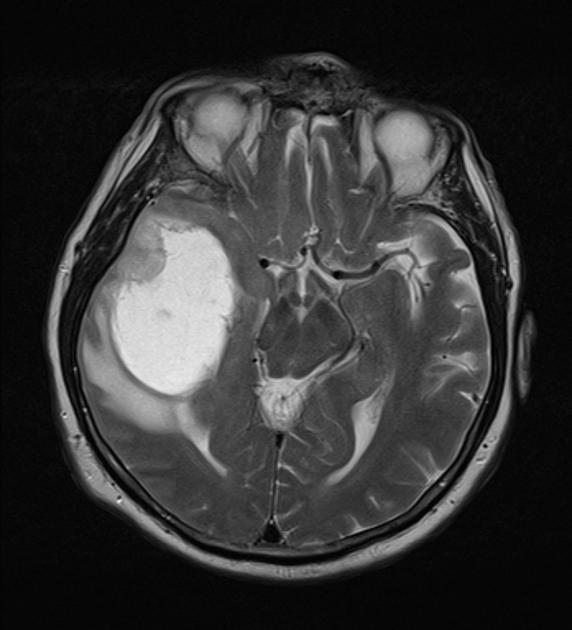
T2/FLAIR
hyperintense
surrounded by vasogenic edema
flow voids are occasionally seen
lacks T2/FLAIR "mismatch" sign
-
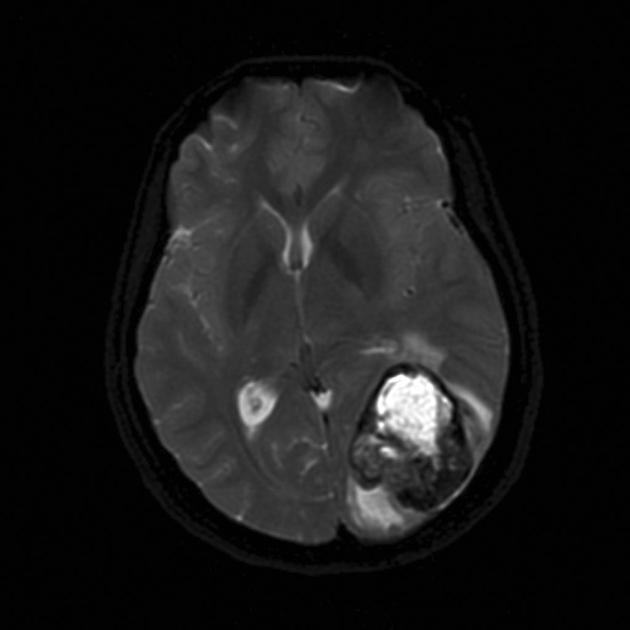
GE/SWI
susceptibility artifact on SWI, also called “intratumoral susceptibility signals”, are usually due to microvascular proliferation and microhemorrhage 33
susceptibility artifact due to calcification are rare
-
low-intensity rim from blood products 6
incomplete and irregular in 85% when present
mostly located inside the peripheral enhancing component
absent dual rim sign
-
DWI/ADC
-
solid component
elevated signal on DWI is common in the solid/enhancing component
ADC values are typically similar to normal white matter (745 ± 135 x 10-6 mm2/s 13), but significantly lower than surrounding vasogenic edema which has facilitated diffusion
-
central necrotic component
facilitated diffusion (ADC values >1000 x 10-6 mm2/s)
care must be taken in interpreting cavities with blood product
-
MR perfusion: rCBV elevated compared to lower grade tumors and normal brain
-
MR spectroscopy
-
typical spectroscopic characteristics include
choline: increased
lactate: increased
lipids: increased
NAA: decreased
myo-inositol: decreased
-
PET
PET demonstrates the accumulation of FDG (representing increased glucose metabolism) which typically is greater than or similar to metabolism in grey matter.
Radiogenomics
A number of features are seen to correlate with molecular marker status, such as MGMT promoter methylation, which typically demonstrates:
high ADC values
limited surrounding edema
low CBV
This has a sensitivity of 79% (95% CI, 72-85%) and specificity of 78% (95% CI, 71-84%) 19.
Radiology report
When reporting a new diagnosis of a mass that is likely a glioblastoma, it is useful to include:
-
morphology
size in three dimensions
presence and degree of central necrosis
non-enhancing tumor involving cortex, deep grey or white matter: look at ADC for lower values
-
relationship to/involvement of
major white matter tracts
large vessels
-
extension
across midline
into brainstem
subependymal spread
CSF dissemination
Treatment and prognosis
Surgery
Maximal safe resection remains an important first step, not only debulking the tumor and reducing symptoms of raised intracranial pressure but also providing tissue for formal diagnosis and assessment of molecular features.
Traditionally the focus of resection has been removal of all enhancing tissue. This has been shown to result in improved survival 25. More recently, there is increased interest in "supramaximal resection" whereby not only is the component that is contrast-enhancing resected, but also adjacent non-enhancing tumor 25.
More complete surgical resection can be aided by the use intraoperative MRI and 5-ALA fluorescence-guided surgery 26.
Adjuvant chemoradiotherapy
Following surgery, postoperative adjuvant radiotherapy and chemotherapy (temozolomide) is the most common treatment (Stupp protocol). Other, often second-line, therapies include lomustine (in combination with temozolamide in patients with MGMT methylated tumors), antiangiogenesis agents (e.g. bevacizumab), immunotherapy, and CAR-T cell therapy 28,29.
Although in individuals less than 70 years of age a standard Stupp protocol is usual, in older individuals the optimum treatment regimen is less well established 15,21. This is particularly the case in the very elderly or those with significant comorbidities 21. In such cases, surgical resection has less marked survival benefit. Radiotherapy is usually administered as a shorter course (e.g. 25-40 Gy in 5-15 daily fractions, rather than 60 Gy over 6 weeks), but even in this setting adding temozolomide significantly increases survival, especially in MGMT methylated (inactive) tumors 15,21.
Prognosis
Despite substantial advances, even in the best-case scenario, glioblastoma carries a poor prognosis with a median survival of <2 years 15.
Negative prognostic factors include:
increased necrosis 10
greater enhancement 10
deep location (e.g. thalamus)
MGMT not-methylated
increased age
lower pre-diagnosis functional status (e.g. ECOG performance status)
TERT promoter mutation (controversial) 31
EGFR amplification 30-32
combined chromosome 7 gain/chromosome 10 loss 30,32
Follow-up
Glioblastomas should be followed up closely with MRI.
Immediate post-operative imaging
Generally a scan is obtained within 24-48 hours of surgery to assess residual disease. Early scanning is necessary to avoid post-operative enhancement that can make interpretation difficult. It should be noted, however, that some post-operative enhancement can occur within the first day post surgery 22 and therefore it is essential that these scans are interpreted alongside the pre-operative scan.
Ongoing imaging
Although timing and frequency will vary between institutions and treating surgeons/oncologists typically scans are obtained every 8 to 12 weeks. In individuals who have no residual macroscopic disease and remain stable for a protracted time, the frequency of follow-up imaging can be gradually decreased. In contrast, sometimes it is worthwhile performing an earlier scan to problem-solve ambiguous imaging features.
The primary aims of on-going follow-up are:
identify tumor progression and complications
distinguish tumor progression from pseudoprogression
distinguish pseudoresponse from tumor progression
Response assessment criteria
Glioblastomas have been the subject of close trial scrutiny with many new chemotherapeutic agents showing promise. As such a number of criteria have been created over the years to assess response to treatment. The response assessment in neuro-oncology (RANO) criteria are most widely used. Other historical systems are worth knowing to allow the interpretation of older data. These systems for response criteria for first-line treatment of glioblastomas include 9:
RANO criteria (most commonly used today)
History and etymology
The original term glioblastoma multiforme was coined in 1926 by Percival Bailey and Harvey Cushing; the suffix multiforme was given to describe the various appearances of hemorrhage, necrosis, and cysts.
Differential diagnosis
General imaging differential considerations include:
-
astrocytoma, IDH-mutant WHO CNS grade 4
may appear very similar/indistinguishable
generally younger patients
T2/FLAIR mismatch sign is common and highly specific 23
-
may look identical
both may appear multifocal
metastases usually are centered on grey-white matter junction and spare the overlying cortex
rCBV in the "edema" will be reduced
-
should be considered especially in patients with AIDS, as in this setting central necrosis is more common
otherwise usually homogeneously enhancing
-
central restricted diffusion is helpful, however, if glioblastoma is hemorrhagic then the assessment may be difficult
presence of smooth and complete SWI low-intensity rim 6
presence of dual rim sign 6
-
can appear similar
often has an open ring pattern of enhancement
usually younger patients
-
subacute cerebral infarction
history is essential in suggesting the diagnosis
should not have elevated choline
should not have elevated rCBV
-
especially in patients with AIDS












 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.