Hypersensitivity pneumonitis, previously known as extrinsic allergic alveolitis, represents a group of immune-mediated pulmonary disorders characterized by an inflammatory and/or fibrotic reaction affecting the lung parenchyma and small airways.
Its diagnosis relies on a constellation of findings: exposure to an offending antigen, characteristic signs and symptoms, abnormal chest findings on physical examination, and abnormalities on pulmonary function tests and radiographic evaluation.
On this page:
Terminology
Hypersensitivity pneumonitis can be categorized into two phenotypic subtypes 13,14:
In patients where an inhaled antigen exposure has not been identified the terms cryptogenic hypersensitivity pneumonitis or hypersensitivity pneumonitis of undetermined cause have been used 14.
Previously, hypersensitivity pneumonitis had been categorized into 5
However this categorization is no longer in favor given the difficulty in delineating between these particular subtypes 14.
Epidemiology
Incidence varies widely based on climate, geography, and occupational or environmental exposures although is estimated at ~0.6 (range 0.3-0.9) per 100,000 population 14.
Diagnosis
A lack of standardized diagnostic criteria for hypersensitivity pneumonia, particularly the fibrotic subtype, remains problematic. A multidisciplinary approach is recommended. A combination of HRCT findings, exposure history, bronchoalveolar lavage lymphocytosis and histopathology can be used to determine the likelihood of hypersensitivity pneumonitis 14.
Clinical presentation
The onset of symptoms may be acute (weeks-months) or can be insidious (month-to-years of gradually worsening symptoms) 14. Common symptoms include dyspnea and cough with less common symptoms being weight loss, fever/chills, malaise, chest tightness and wheezing 14.
Clinical examination may demonstrate mid-inspiratory squeaks and finger clubbing. There is a restriction pattern with decreased diffusing capacity on pulmonary function tests 3,14.
Pathology
Etiology
More than 200 different antigens have been associated with the development of hypersensitivity pneumonitis. Antigen exposure may be domestic, industrial and/or recreational and there may be more than one antigen exposure 14:
-
microbes
fungi/molds, e.g. mushrooms, Aspergillus, Cryptococcus
yeasts, e.g. candida
bacteria, e.g. Pseudomonas
protozoa, e.g. Amoebae
nematodes
mites, e.g. Acarus siro
-
proteins
animal proteins, e.g. animal fur dust, avian droppings/feathers
plant proteins, e.g. wood particles, grain flour
-
inorganic particular matter
chemicals, e.g. isocyanate found in paint hardener
pharmaceuticals, e.g. antibiotics such as penicillin, cephalosporin 15, immunosuppressants such as sirolimus/everolimus 8
The triggering particles are usually in the range of 1-5 micrometers in size 5. In ~40% (range 30-50%) the inciting agent may not be identified 14.
Depending on the type of precipitant, numerous other more precipitant-specific clinical terms have been used such as:
bird fancier's lung (inclusive of pigeon fancier's lung)
Microscopic appearance
The histopathologic process consists of chronic inflammation of the bronchi and peribronchiolar tissue, often with poorly defined granulomas and giant cells in the interstitium or alveoli. Fibrosis and emphysema may develop later on.
Most cases of hypersensitivity pneumonitis, whether acute or insidious, include the following four histologic features in variable amounts and combinations 3.
cellular bronchiolitis: chronic inflammatory cells lining the small airways, sometimes with resultant epithelial ulceration
diffuse chronic interstitial inflammatory infiltrates: primarily consisting of lymphocytes and plasma cells but often including eosinophils, neutrophils, and mast cells
poorly circumscribed interstitial non-necrotizing (non-caseating) granulomas: consisting of lymphocytes, plasma cells, and epithelioid histiocytes, with or without giant cells
individual giant cells in the alveoli or interstitium
Smoking is protective against hypersensitivity pneumonitis, presumably by the inhibitory action of nicotine on macrophage activation and lymphocyte proliferation and function 9. However, when smokers do develop hypersensitivity pneumonitis, it is more commonly fibrosing disease with a worse prognosis 10.
Subtypes
Hypersensitivity pneumonitis can be categorized into two subtypes based on the absence/presence of fibrosis given this is a determining factor in prognosis 13,14:
non-fibrotic hypersensitivity pneumonitis: purely inflammatory
fibrotic hypersensitivity pneumonitis: mixed inflammatory/fibrotic or purely fibrotic
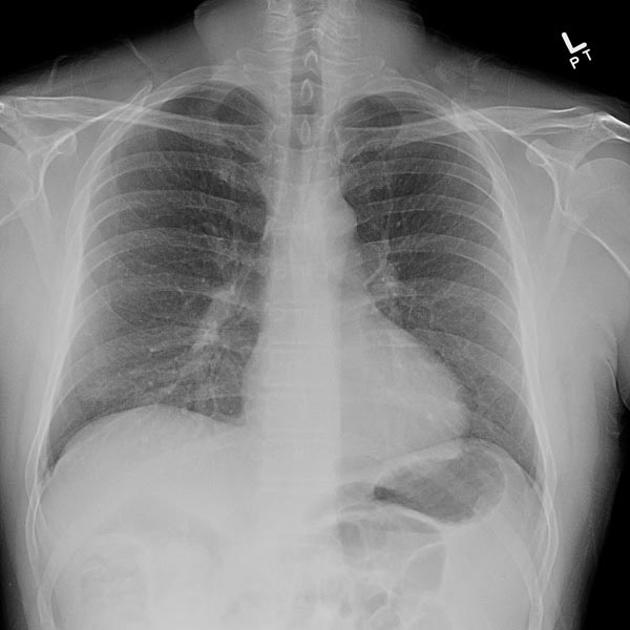
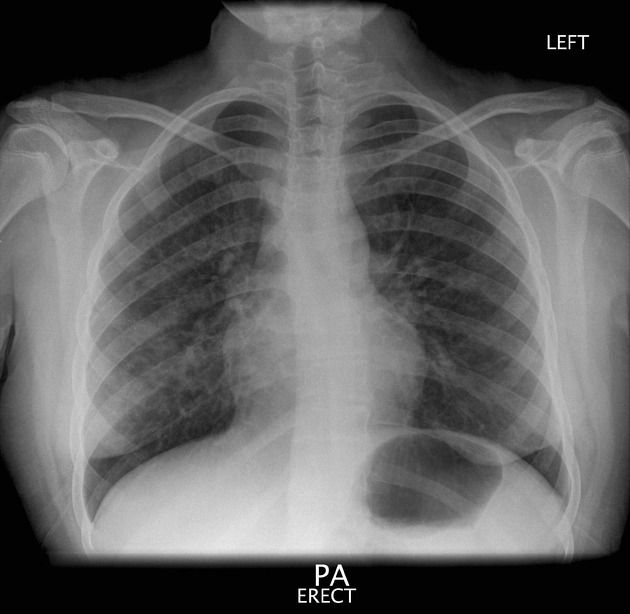
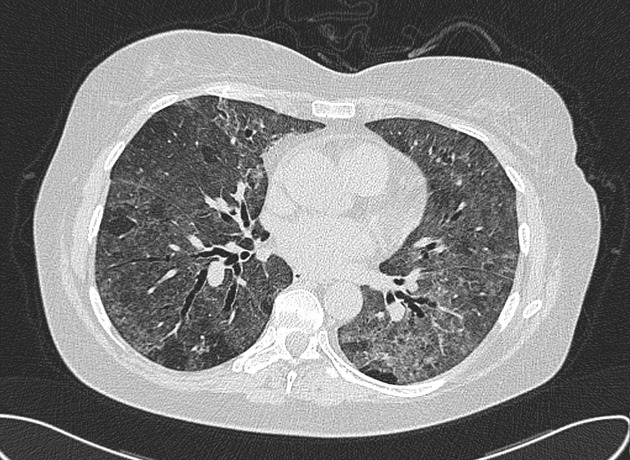
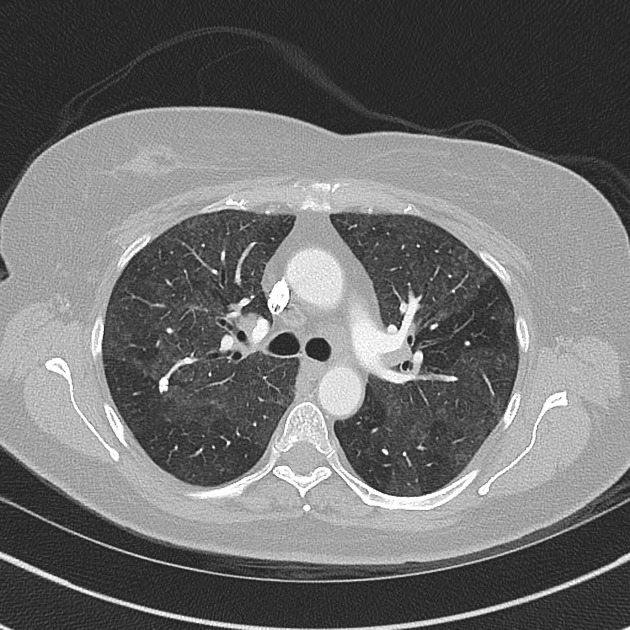
Radiographic features
HRCT chest is the main imaging technique for hypersensitivity pneumonitis 14. In population-based studies, the sensitivity of chest radiographs is relatively low 1. Many patients have normal radiographs 3.
Plain radiograph
Abnormal plain radiographic findings may be observed in some patients can include 3
numerous poorly defined small (<5 mm) opacities throughout both lungs, sometimes with sparing of the apices and bases
airspace disease: usually seen as ground-glass opacities (can be patchy or diffuse, resembling pulmonary edema) or, more rarely, as consolidation
a pattern of fine reticulation may also occur
zonal distribution is variable from patient to patient and may even show temporal variation within the same patient
Later stages
when fibrosis develops: there may be a reticular pattern and honeycombing, which sometimes are more severe in the upper lobes than in the lower ones
volume loss may occur: particularly in the upper lungs, and peribronchial thickening may be visible
cardiomegaly may develop as a result of cor pulmonale
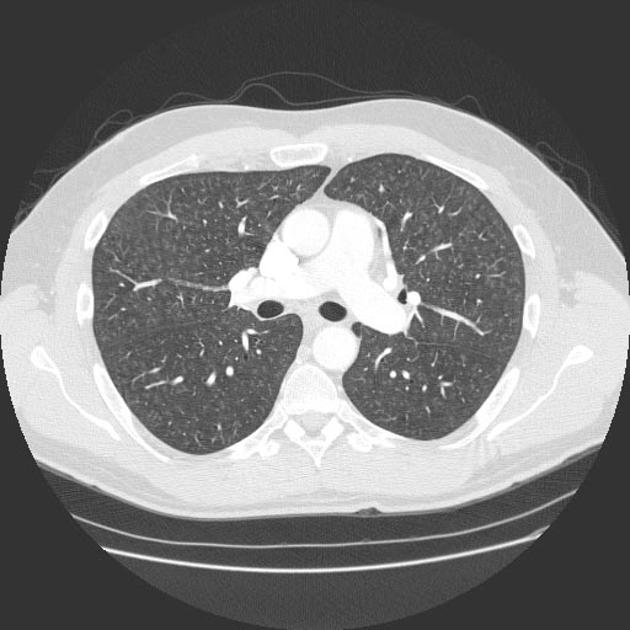
CT
The HRCT features for each hypersensitivity pneumonitis subtype is able to be described as 14:
typical hypersensitivity pneumonitis
compatible with hypersensitivity pneumonitis
indeterminate for hypersensitivity pneumonitis
Non-fibrotic hypersensitivity pneumonitis
-
typical: at least one abnormality of the parenchyma and small airways
parenchyma: ground-glass opacity (GGO), mosaic attenuation
small airways: ill-defined centrilobular nodules, air trapping
diffuse distribution +/- basal sparing
-
compatible: non-specific changes reported in hypersensitivity pneumonitis
parenchyma: uniform and subtle GGOs, airspace consolidation, pulmonary cysts
diffuse distribution (although variant distributions of basal predominant or peribronchovascular) 14
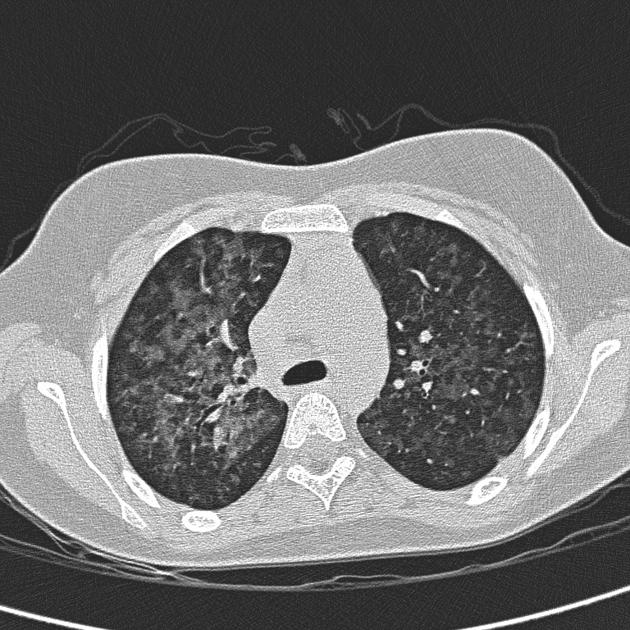
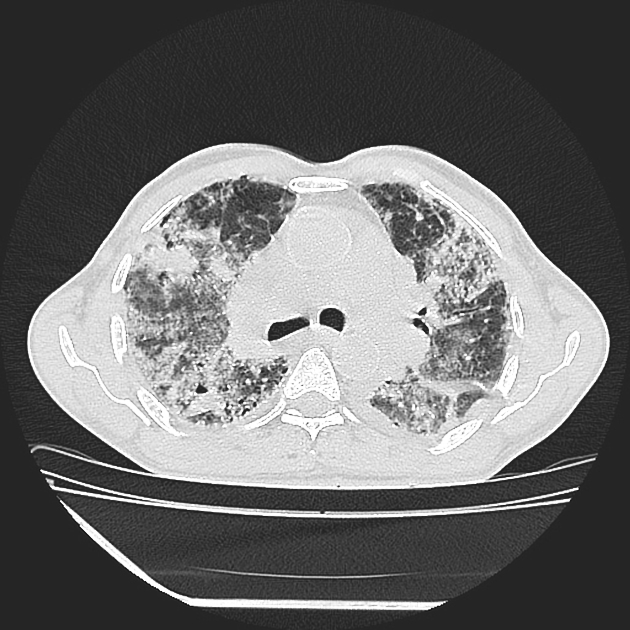
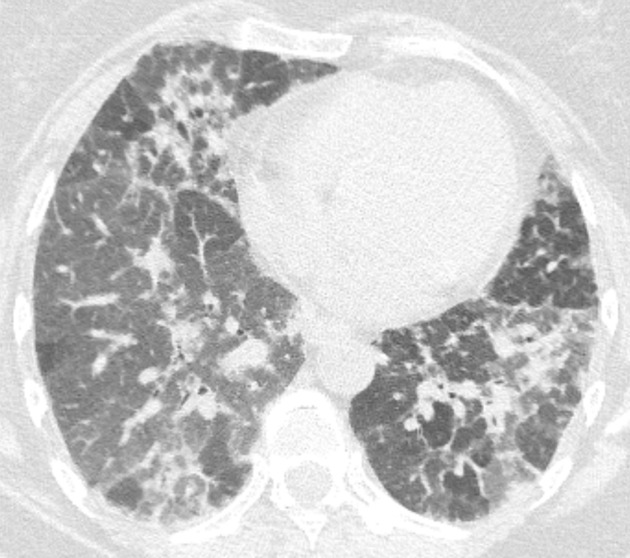
Fibrotic hypersensitivity pneumonitis
The three-density pattern on inspiratory CT has high specificity for fibrotic HP 16. Bilateral mosaic attenuation affecting 3 or more lobes, (more than 5 lobules in each lobe) distinguishes fibrotic HP from IPF which may contain more limited areas of air-trapping 16.
-
typical
coarse reticulations with lung distortion +/- non-predominate traction bronchiectasis and honeycombing
-
distribution may be
random
mid-zone predominant
relative lower zone sparing
-
small airways disease
ill-defined centrilobular nodules and/or GGOs
mosaic attenuation, three-density pattern (head cheese sign) and/or air trapping
-
compatible
extensive GGOs with subtle lung fibrotic changes
-
variant distribution of lung fibrosis
peribronchovascular
subpleural
upper zones
-
small airways disease
ill-defined centrilobular nodules
three-density pattern and/or air trapping
-
indeterminate
UIP pattern alone (i.e. no variant distribution or small airways disease)
indeterminate or probable UIP pattern
Other associated features of both subtypes include ref:
small volume mediastinal lymphadenopathy (generally 10-20 mm in short-axis diameter)
occasional pulmonary arterial enlargement
with developing fibrosis, there can be reticulation, mainly in the middle portion of the lungs or fairly evenly throughout the lungs but with relative sparing of the extreme apices and bases
Treatment and prognosis
Removal of the precipitant is often the key to management. Glucocorticoids may play a role, particularly when symptoms persist despite removal of the precipitant.
Differential diagnosis
Due to a variable radiographic presentation, it may not be meaningful to give a differential diagnosis for hypersensitivity pneumonitis per se. It is better to refer to the differential for a particular radiographic feature:
differential for centrilobular nodular opacities
differential for ground-glass opacities




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.