Cholangiocarcinomas (commonest type of bile duct cancers) are malignant epithelial tumors arising from the biliary tree, excluding the gallbladder or ampulla of Vater. Cholangiocarcinoma is the third most common primary hepatobiliary malignancy after hepatocellular carcinoma (HCC) and gallbladder cancer 23. They tend to have a poor prognosis and high morbidity.
On this page:
Epidemiology
Although overall cholangiocarcinoma is rare, there are significant regional variations in incidence with much higher rates seen in Southeast Asia and the Middle East 2. The incidence ranges from 0.3 to 6 per 100,000 inhabitants per year 14.
They usually present in the elderly, with a mean age of 65 years 7. There may be a slight male predilection.
Cholangiocarcinomas correspond to ~15% of all primary liver tumors and to ~3% of all gastrointestinal malignancies 14.
Risk factors
A number of risk factors for cholangiocarcinoma have been identified, and bile stasis and chronic inflammation of the biliary epithelium are identified as common features among many of them 1,2,9,14:
Caroli disease / choledochal cysts: lifetime risk of 10-15% 2
choledocholithiasis: more than cholelithiasis 10,11
primary sclerosing cholangitis (PSC): especially in Western countries
recurrent pyogenic cholangitis: especially in Southeast Asia
cirrhosis 14
toxins: e.g. thorotrast, dioxin, polyvinylchloride, heavy alcohol use
viral infections: e.g. HIV, hepatitis B, hepatitis C, EBV
liver fluke infestation: Opisthorchis spp. and Clonorchis spp. 18,20
hepatolithiasis 18,21
Regarding inflammatory bowel disease, cholangiocarcinoma develops in one in every 100 to 200 patients, which means they have a 4 times greater risk than IBD-free patients. Individuals with ulcerative colitis are at higher risk of having cholangiocarcinoma in comparison to those with Crohn disease 17.
Clinical presentation
Typically, the presentation is with painless jaundice.
Pathology
Location
Cholangiocarcinomas may be classified anatomically as follows 16:
intrahepatic (10% of cases)
-
extrahepatic
-
perihilar (70%): occur between the secondary biliary radicles and proximal to the cystic duct insertion
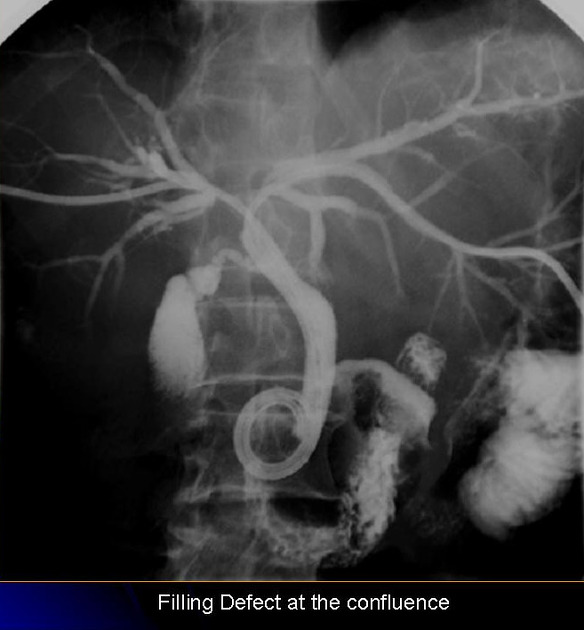
tumors involving the confluence of the right and left hepatic ducts are frequently referred to as Klatskin tumors, although use of the eponym is going out of favor in both the radiology and the clinical literature 22
distal (20%): distal to the cystic duct insertion
-
NB: There is variability in nomenclature in the literature, as perihilar cancers often span the vague and variable boundary between intrahepatic and extrahepatic bile ducts. For example, intrahepatic cholangiocarcinomas are sometimes subclassified into peripheral and hilar types 16.
Some institutions are discouraging the use of the terms Klatskin tumor and extrahepatic cholangiocarcinoma in favor of perihilar and distal cholangiocarcinoma in an attempt to standardize the classification and provide useful clinical information. Indeed, the International Liver Cancer Association guidelines on "Diagnosis and management of intrahepatic cholangiocarcinoma" published in 2014 discourage using the term Klatskin tumor altogether 22.
Perihilar cholangiocarcinomas are commonly subclassified by their anatomic extent according to the Bismuth-Corlette classification (described separately).
Classification
Prognostic staging uses the TNM system, depending on location:
The previously mentioned Bismuth-Corlette anatomic classification is used for operative planning but is limited in predicting prognosis 16.
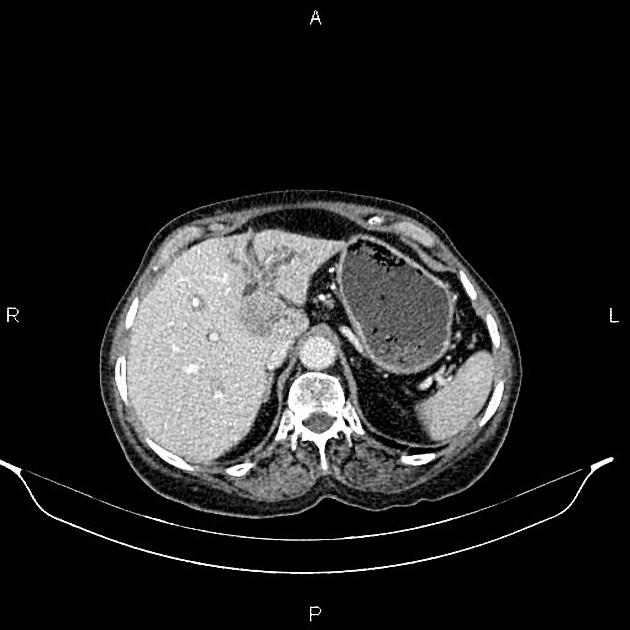
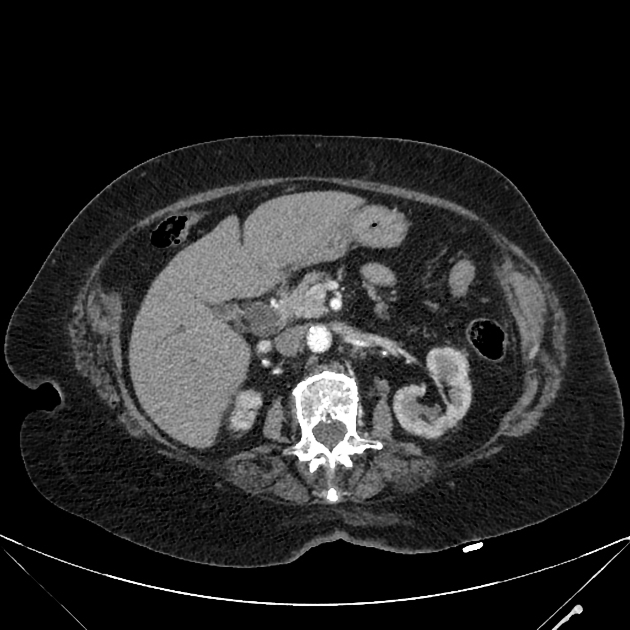
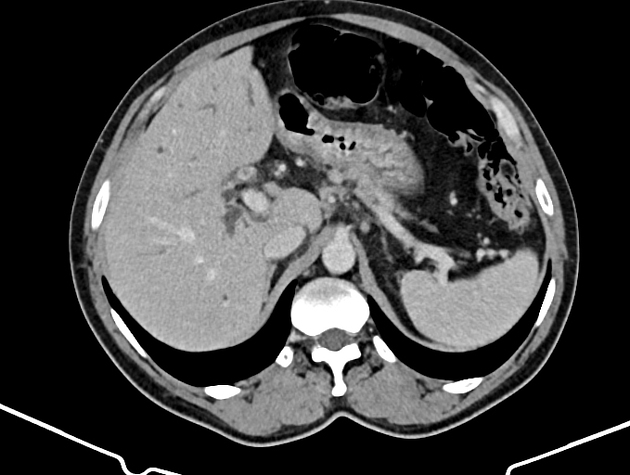
Macroscopic appearance
Cholangiocarcinomas are typically sclerotic masses without hemorrhage or macroscopic necrosis 2. In general, the active tumor is at the periphery, with the central portions replaced by fibrosis, accounting for the capsular retraction that may be seen in intrahepatic tumors.
The macroscopic appearance is further described according to the Liver Cancer Study Group of Japan classification 15,16:
mass-forming: definite mass in the liver parenchyma
periductal-infiltrating: extends longitudinally along the bile duct, often causing peripheral bile duct dilation
intraductal: proliferates in the lumen of the bile duct-like a papilla or tumor thrombus
Mass-forming
Intrahepatic exophytic nodular (peripheral) tumors are most commonly of the mass-forming type 3. They demonstrate variable amounts of central fibrosis, usually marked.
Periductal infiltrating
Periductal infiltrating intrahepatic tumors are most common at the hilum (comprise over 70% of hilar-perihilar cholangiocarcinomas), known as Klatskin tumors 3, but can also be seen in combination with mass-forming tumors within the liver. Growth along the walls of the duct may narrow or dilate the duct.
Intraductal
Intraductal tumors comprise 8-18% of resected cholangiocarcinomas 3 and a much smaller number of all cholangiocarcinomas (as most are inoperable). They are characterized by alterations in duct caliber, usually duct ectasia with or without a visible mass. If a mass is visible it may be mural or polypoid in shape 2. The duct dilatation is thought to be due to abundant mucin production. This entity is thought to be similar to the pancreatic intraductal papillary mucinous neoplasms (IPMN).
Microscopic appearance
Histologically, cholangiocarcinomas are divided into well, moderately, and poorly differentiated adenocarcinomas 2. In specimens of bile ducts from patients with hepatolithiasis, biliary intraepithelial neoplasia (BilIN) is a common finding and is considered to be a precursor lesion of cholangiocarcinoma. It is typically a microscopic lesion with a flat or micropapillary dysplastic epithelium. It is synonymous with carcinoma in situ 2.
Radiographic features
Ultrasound
The appearance will vary according to the growth pattern.
Mass-forming: tumors will be a homogeneous mass of intermediate echogenicity with a peripheral hypoechoic halo of compressed liver parenchyma. They tend to be well delineated but irregular in outline and are often associated with capsular retraction 2 which, if present, helps distinguish cholangiocarcinomas from other hepatic tumors.
Periductal infiltrating: tumors typically are associated with altered caliber bile duct (narrowed or dilated) without a well-defined mass.
Intraductal: tumors are characterized by alterations in duct caliber, usually duct ectasia with or without a visible mass. If a polypoid mass is seen, it is usually hyperechoic compared to surrounding liver 2.
Contrast-enhanced ultrasound
Contrast-enhanced ultrasound may aid with the diagnosis of cholangiocarcinoma 8:
-
arterial phase
peripheral irregular rim-like enhancement
heterogeneous central hypoenhancement
-
portal venous phase / delayed phase
decreased echogenicity relative to background liver ("wash out")
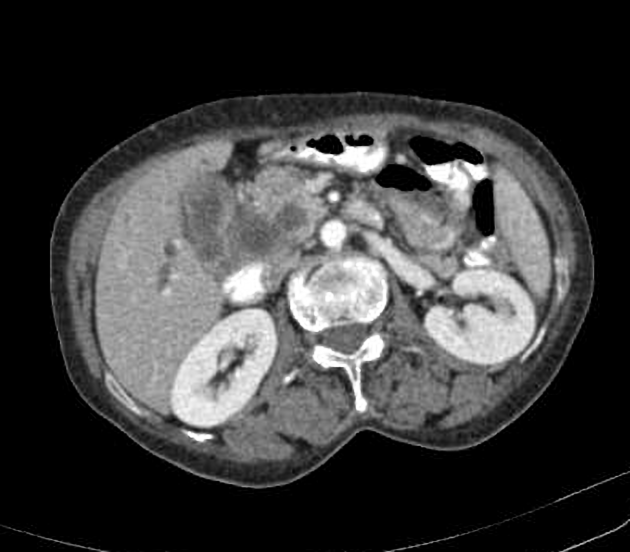
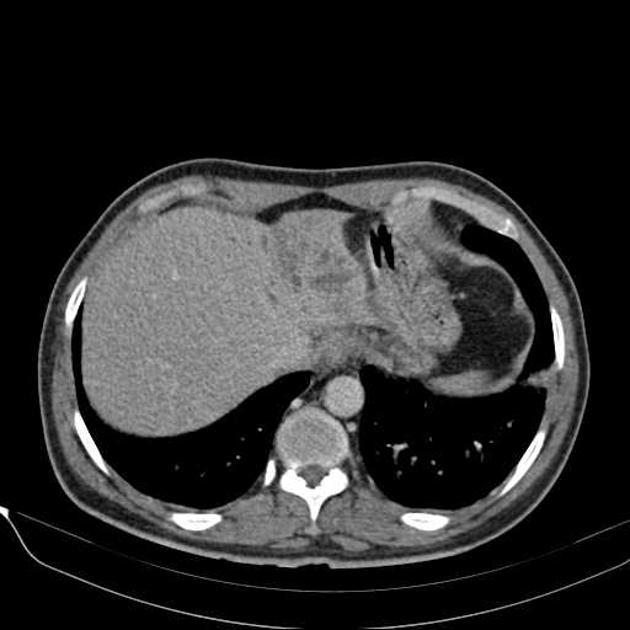
CT
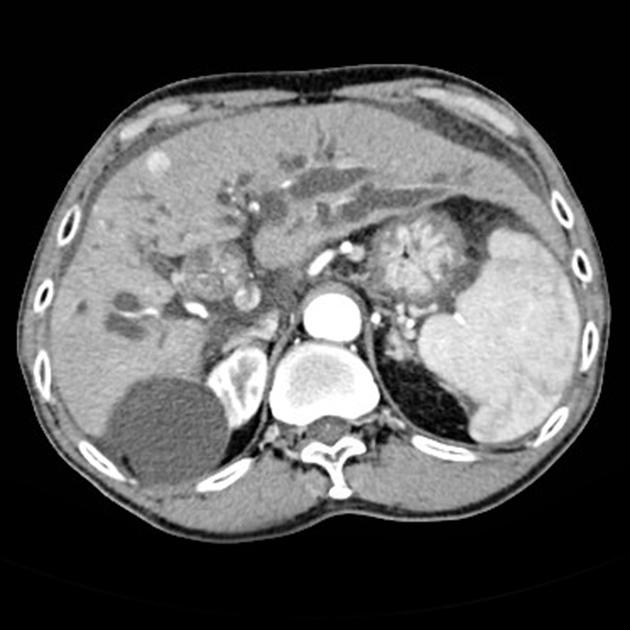
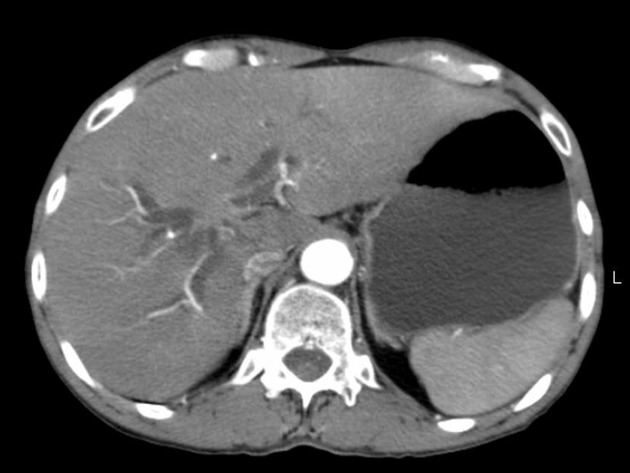
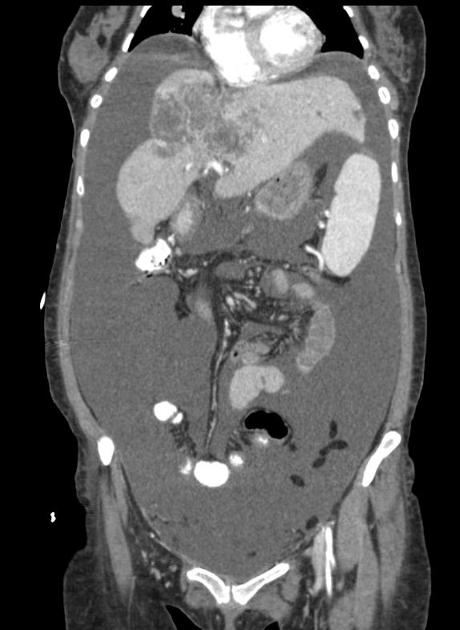
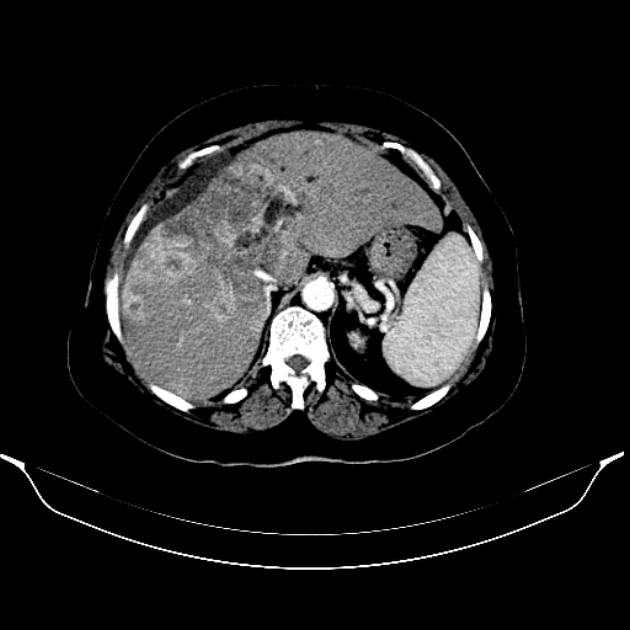
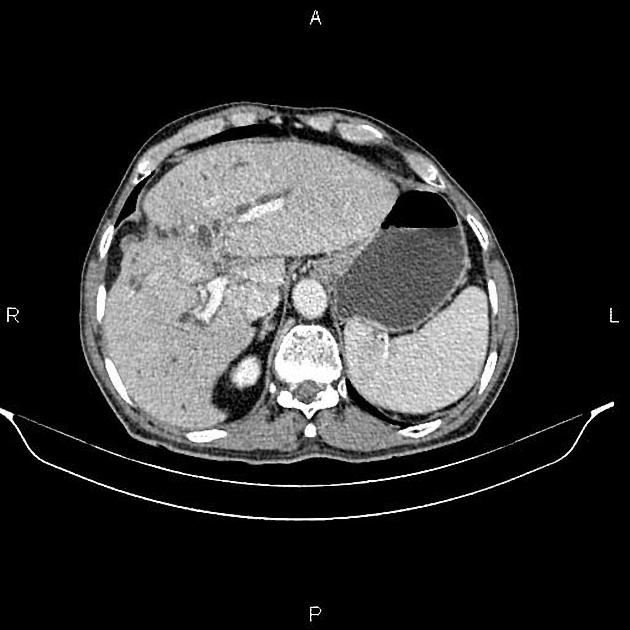
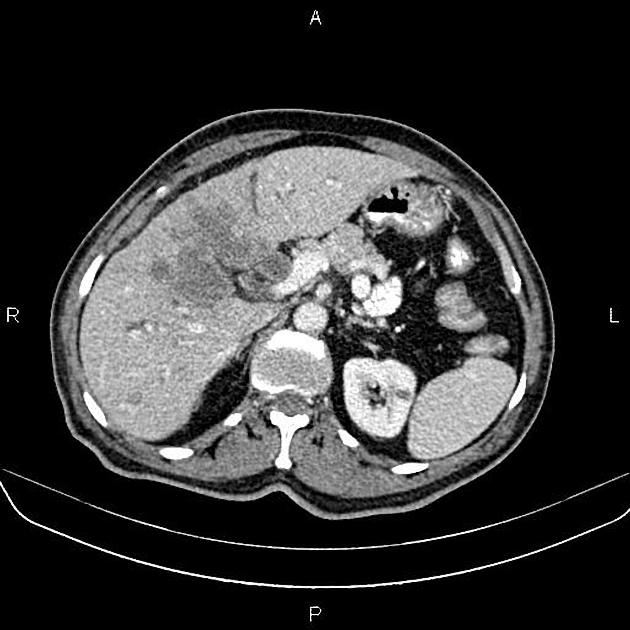
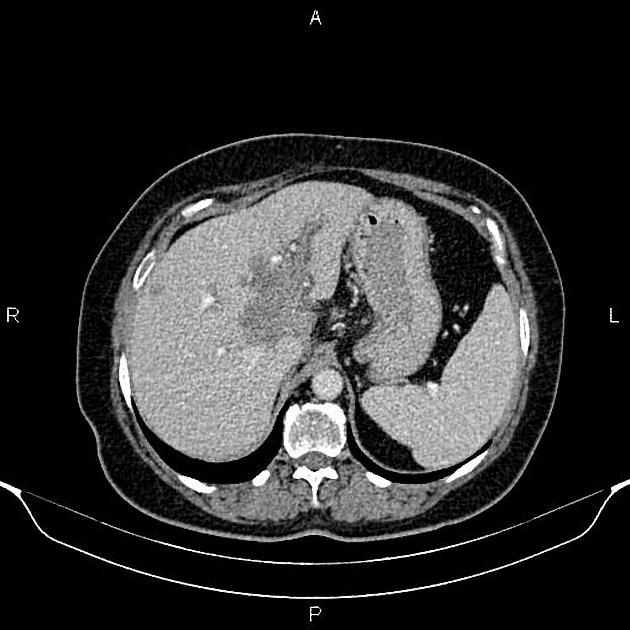
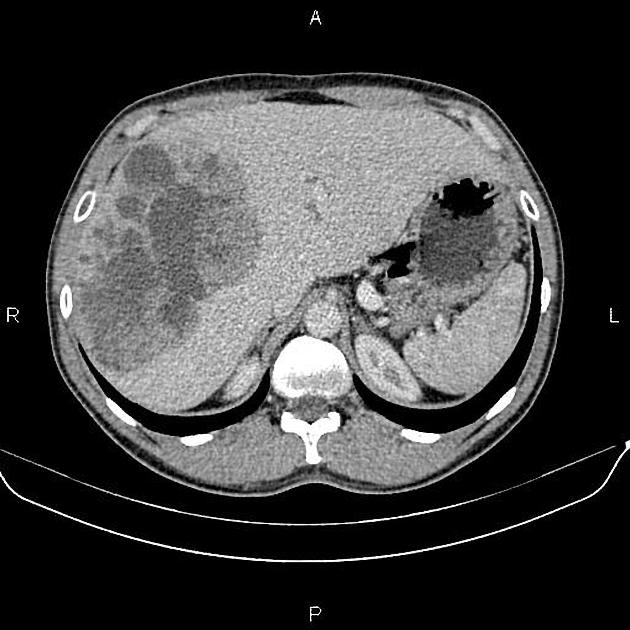
Mass-forming: these are typically homogeneously low in attenuation on non-contrast scans, and demonstrate heterogeneous minor peripheral enhancement with gradual centripetal enhancement 2,3. The rate and extent of enhancement depend on the degree of central fibrosis 2. Again, capsular retraction may be evident. Calcification may be seen in ~20% 24. The bile ducts distal to the mass are typically dilated.
Although narrowing the portal veins, or less frequently hepatic veins, is seen, unlike hepatocellular carcinoma, cholangiocarcinoma only rarely forms a tumor thrombus 2.
Lobar or segmental hepatic atrophy is usually associated with vascular invasion 6.
Periductal infiltrating: intrahepatic tumors appear as regions of duct wall thickening or of the periductal parenchyma, with altered caliber of the involved duct (usually narrowed). These are most common at the hepatic hilum. They tend to be longer than benign strictures (i.e. approximately 20 mm in length) and show contrast enhancement. There is usually some proximal (i.e. peripheral) dilatation of the biliary tree.
Intraductal: these are characterized by alterations in duct caliber, usually duct ectasia with or without a visible mass. If a polypoid mass is seen, it is hypoattenuating on pre-contrast imaging and demonstrates enhancement 2.
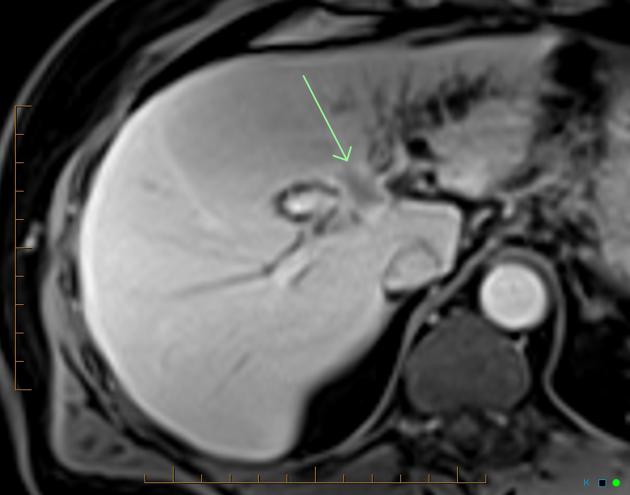
MRI
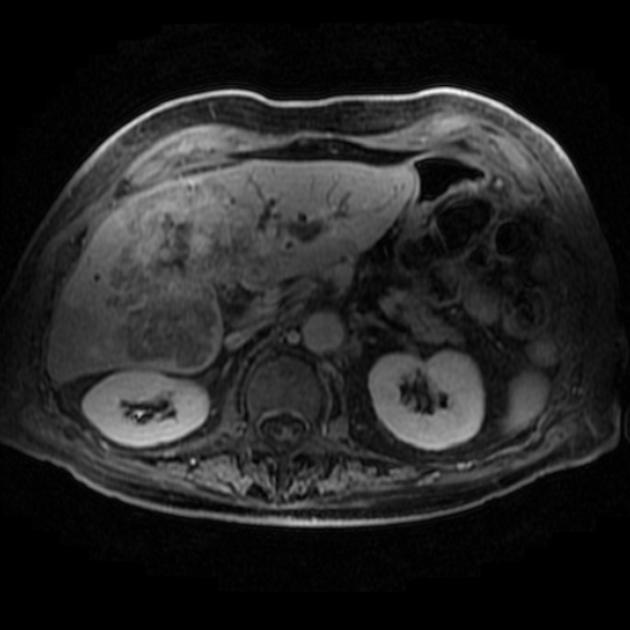
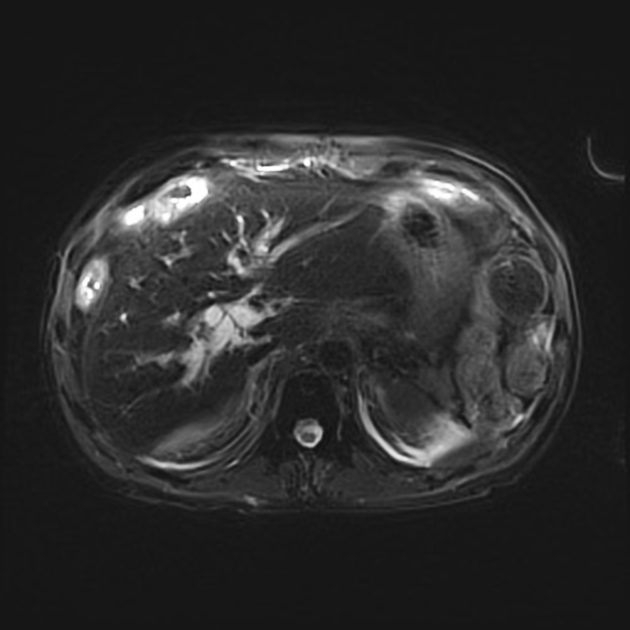
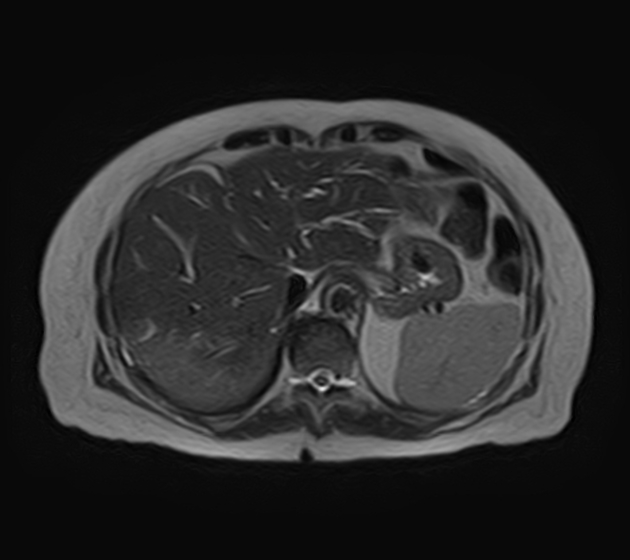
MRI is the imaging modality of choice as it can best visualize all three of the tumor mass, the biliary ducts, and the blood vessels, all of which are essential for determining resectability (see below). Appearances on MR are similar to those described above for CT, except that MR is more sensitive to contrast enhancement 3 and bile duct visualization.
DWI/ADC: a peripherally hyperintense "target" appearance on DWI favors cholangiocarcinoma over hepatocellular carcinoma
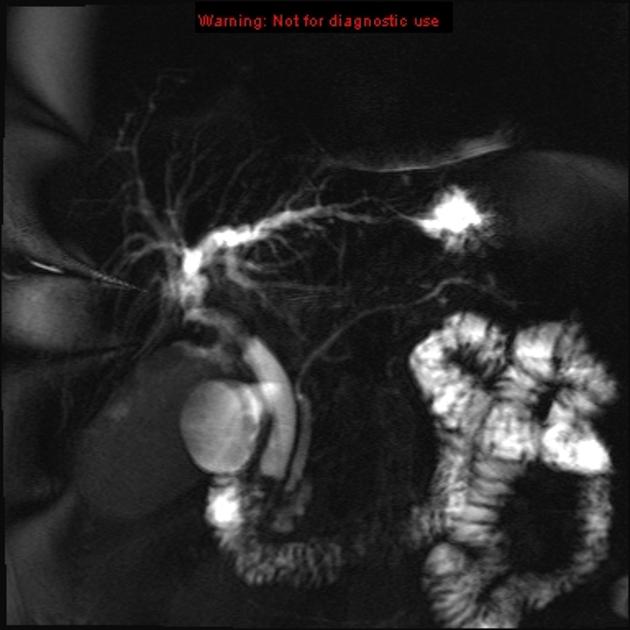
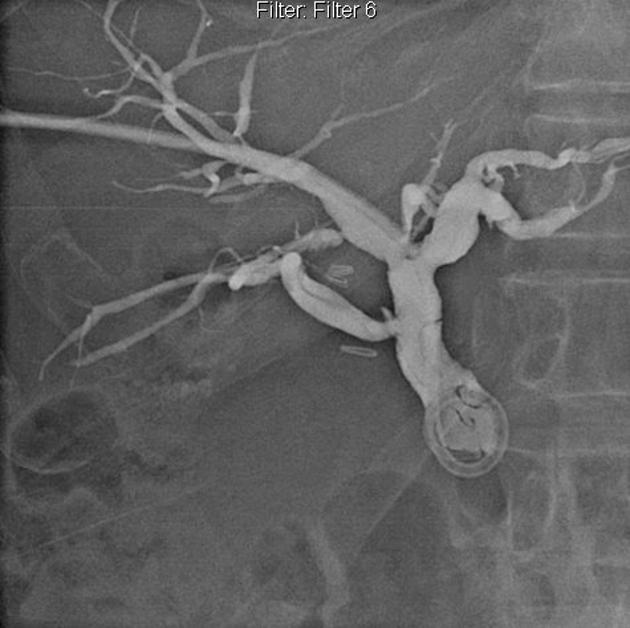
Direct cholangiography
Direct cholangiography is a blanket term for any imaging obtained with intra-biliary tree contrast and includes:
All these modalities not only allow evaluation of the biliary tree but are invaluable in planning treatment and assessing for resectability.
Radiology report
The following reporting checklist pertains to hilar/perihilar cholangiocarcinoma, as it is anatomically close to the large bile ducts and blood vessels, crucial to the determination of resectability:
-
bile ducts (see Bismuth-Corlette classification)
is the tumor confined to the common or hepatic bile duct?
is there extension to the right or left hepatic duct or both?
does the tumor involve second-order radicles and on which side?
portal vein: does the tumor abut/encase the main/right/left portal vein and to what extent?
-
hepatic artery
is the common hepatic artery/hepatic artery proper involved and to what extent?
is the right/left hepatic artery involved and to what extent?
lymph nodes: enlarged regional (N1) or distant (N2) lymph nodes?
assess for distal metastases
Treatment and prognosis
The most important factor in prognosis is whether or not the tumor can be resected. Unfortunately, when discovered, most cases are too advanced for curative resection. Even with resection, the prognosis is poor, with a 5-year survival of only 10-44% 4, with the prognosis favoring extrahepatic tumors (around 30% 5-year survival vs 15% for intrahepatic tumors).
The pattern of metastatic spread includes 1:
intrahepatic vascular involvement with numerous local metastases
regional lymph nodes (50% at autopsy)
-
hematogenous (50% at autopsy)
lungs
bones, especially vertebrae
adrenal glands
brain
Surgical resectability
An increase in margin-negative resection rates and, hence, survival can be achieved by resection of the ipsilateral hepatic lobe. In the interest of leaving the patient with a large enough contralateral lobe, portal vein branch embolization of the lobe intended for resection 4-6 weeks before surgery can induce hypertrophy of the contralateral lobe. It should be noted that when attempting resection, tumor size in itself is unimportant.
A hilar-perihilar tumor is considered unresectable in the following cases 12:
Bismuth type IV: bilateral secondary biliary radicle involvement
main portal vein encasement/occlusion
atrophy of a liver lobe with contralateral portal vein or hepatic artery encasement
atrophy of a liver lobe with contralateral secondary biliary radicle involvement
involvement of both hepatic arteries
Differential diagnosis
The differential diagnosis depends on whether the tumor is intrahepatic or extrahepatic and on the growth pattern.
For a mass-forming cholangiocarcinoma consider the following:
-
central necrosis (high T2 signal) is more common
-
hepatocellular carcinoma (HCC)
tumor thrombus more common
capsular retraction uncommon
may appear very similar
other primary liver tumors
For a periductal infiltrating cholangiocarcinoma consider the following:
-
usually short-segment
regular margin, but there are exceptions to this
symmetric narrowing
no ductal enhancement
no lymph node enlargement
no periductal soft-tissue mass
periportal lymphangitic metastasis 2
For an intraductal cholangiocarcinoma consider:
-
intraductal invasion by hepatocellular carcinoma (HCC)
extraductal mass
-
no enhancement
higher attenuation
-
biliary cystadenoma or cystadenocarcinoma
intratumoral cysts do not communicate with the biliary tree




 Unable to process the form. Check for errors and try again.
Unable to process the form. Check for errors and try again.